Although the precise mechanism of action for valproic acid is unknown, its antiepileptic effect is thought to be due to its ability to increase concentrations of the neuroinhibitor γ-aminobutyric acid (GABA), to potentate the postsynaptic response to GABA, or to exert a direct effect on cellular membranes.11,12
THERAPEUTIC AND TOXIC CONCENTRATIONS
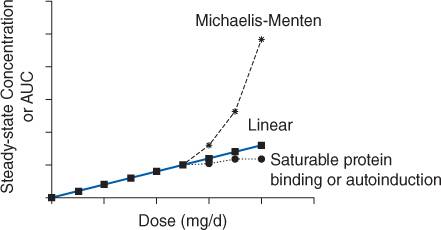
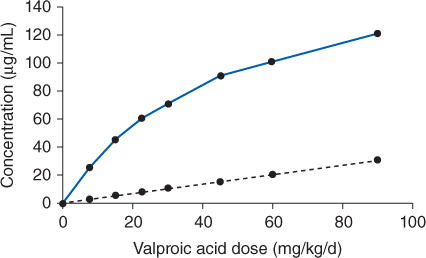
The generally accepted therapeutic range for total valproic acid steady-state concentrations is 50-100 μg/mL, although some clinicians suggest drug concentrations as high as 175 μg/mL with appropriate monitoring of serum concentrations and possible adverse effects. Valproic acid is highly protein bound to albumin with typical values of 90%-95%.13,14 Plasma protein binding of valproic acid is saturable within the therapeutic range which results in less protein binding and higher unbound fraction of drug at higher concentrations. The concentration-dependent protein binding of valproic acid causes the drug to follow nonlinear pharmacokinetics (Figure 12-1). This type of nonlinear pharmacokinetics is fundamentally different than that observed during phenytoin administration. Phenytoin hepatic metabolism becomes saturated which causes Michaelis-Menten pharmacokinetics to take place. As a result, when phenytoin doses are increased total and unbound steady-state concentrations increase more than a proportional amount (eg, when the dose is doubled, serum concentrations may increase 3-5-fold or more). In the case of valproic acid, when the dose is increased total drug steady-state concentration increases less than expected, but unbound steady-state drug concentration increases in a proportional fashion (eg, when the dose is doubled, total serum concentration increases 1.6-1.9 times but unbound steady-state serum concentration doubles; Figure 12-2). The pharmacokinetic rational for these changes is explained fully in the Basic Clinical Pharmacokinetic Parameters section found later in this chapter.
FIGURE 12-1 If a drug follows linear pharmacokinetics, Css or AUC increases proportionally with dose resulting in a straight line on the plot. Nonlinear pharmacokinetics occurs when the Css or AUC versus dose plot results in something other than a straight line. If a drug follows Michaelis-Menten pharmacokinetics (eg phenytoin, aspirin), as steady-state drug concentrations approach Km, serum concentrations increase more than expected due to dose increases. If a drug follows nonlinear protein binding (eg, valproic acid, disopyramide) or autoinduction (eg, carbamazepine), total steady-state drug concentrations increase less than expected as dose increases.
FIGURE 12-2 Although total valproic acid concentrations increase in a nonlinear fashion with dosage increases (solid line), unbound, or “free” valproic acid concentrations increase in a linear fashion with dosage increases (dashed line). Valproic acid is a low-extraction ratio drug, and its unbound serum concentrations are only a function of intrinsic clearance (Cl′int): Css, u = (D/τ)/Cl′int, where D is valproic acid dose in mg, τ is the dosage interval in h, and Css, u is the unbound steady-state valproic acid concentration.
Insufficient prospective work has been done to establish the therapeutic range for unbound valproic acid steady-state serum concentrations. As an initial guide, 5% of the lower end and 10% of the upper end of the total concentration therapeutic range is used to construct the preliminary unbound steady-state concentration therapeutic range for valproic acid of 2.5-10 μg/mL. The percent used for each case is the average unbound fraction of drug at the appropriate concentration.
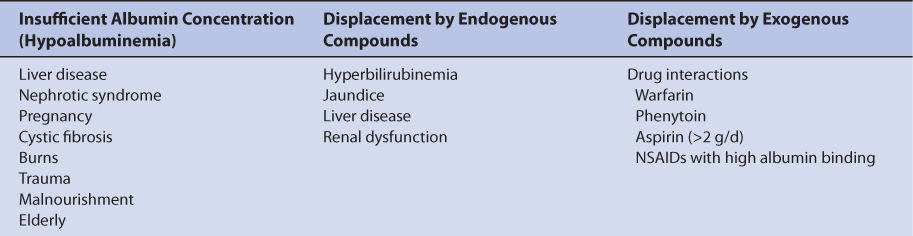
More information is available that identifies the clinical situations where unbound valproic acid serum concentration measurement is useful. As is the case with phenytoin, measurement of unbound valproic acid serum concentrations should be considered in patients with factors known to alter valproic acid plasma protein binding.14–18 These factors fall into three broad categories: (1) lack of binding protein where there are insufficient plasma concentrations of albumin, (2) displacement of valproic acid from albumin-binding sites by endogenous compounds, and (3) displacement of valproic acid from albumin-binding sites by exogenous compounds (Table 12-2).
Low albumin concentrations, known as hypoalbuminemia, can be found in patients with liver disease or the nephrotic syndrome, pregnant women, cystic fibrosis patients, burn patients, trauma patients, malnourished individuals, and the elderly. Albumin concentrations below 3 g/dL are associated with high valproic acid unbound fractions in the plasma. Albumin is manufactured by the liver so patients with hepatic disease may have difficulty synthesizing the protein. Patients with nephrotic syndrome waste albumin by eliminating it in the urine. Malnourished patients can be so nutritionally deprived that albumin production is impeded. Malnourishment is the reason for hypoalbuminemia in some elderly patients, although there is a general downtrend in albumin concentrations in older patients. However, the unbound fraction of valproic acid is higher in elderly patients even if albumin concentrations are within the normal range. While recovering from their injuries, burn and trauma patients can become hypermetabolic and albumin concentrations decrease if enough calories are not supplied during this phase of their disease state. Albumin concentrations may decline during pregnancy as maternal reserves are shifted to the developing fetus and are especially prevalent during the third trimester.
Displacement of valproic acid from plasma protein–binding sites by endogenous substances can occur in patients with hepatic or renal dysfunction. The mechanism is competition for albumin plasma protein–binding sites between the exogenous substances and valproic acid. Bilirubin (a byproduct of heme metabolism) is broken down by the liver, so patients with hepatic disease can have excessive bilirubin concentrations. Total bilirubin concentrations in excess of 2 mg/dL are associated with abnormal valproic acid plasma protein binding. End-stage renal disease patients (creatinine clearance <10-15 mL/min) with uremia (blood urea nitrogen concentrations >80-100 mg/dL) accumulate unidentified compound(s) in their blood that displace valproic acid from plasma protein–binding sites. Abnormal valproic acid binding persists in these patients even when dialysis procedures are instituted.
Valproic acid plasma protein–binding displacement can also occur due to exogenously administered compounds such as drugs. In this case, the mechanism is competition for albumin-binding sites between valproic acid and other agents. Other drugs that are highly bound to albumin and cause plasma protein–binding displacement drug interactions with valproic acid include warfarin, phenytoin, aspirin (>2 g/d), and some highly bound nonsteroidal anti-inflammatory agents.
In the upper end of the therapeutic range (>75 μg/mL) some patients will begin to experience the concentration-dependent adverse effects of valproic acid therapy: ataxia, sedation, lethargy, and tiredness. In many individuals, these side effects dissipate with continued dosing, and slow dosage titration may assist in minimizing these adverse reactions in newly treated patients. Other concentration-related side effects of valproic acid therapy include tremor at concentrations >100 μg/mL, and stupor or coma at concentrations >175 μg/mL. Additionally, valproic acid–associated thrombocytopenia can usually be limited by a decrease in drug dose.
CLINICAL MONITORING PARAMETERS
The goal of therapy with anticonvulsants is to reduce seizure frequency and maximize quality of life with a minimum of adverse drug effects. While it is desirable to entirely abolish all seizure episodes, it may not be possible to accomplish this in many patients. Patients should be monitored for concentration-related side effects (ataxia, sedation, lethargy, tiredness, tremor, stupor, coma, thrombocytopenia) as well as gastrointestinal upset associated with local irritation of gastric mucosa (nausea, vomiting, anorexia).12 Elevated liver function tests, increased serum ammonia, alopecia, and weight gain have been reported during chronic valproic acid treatment. Serious, but rare, idiosyncratic side effects include hepatotoxicity, pancreatitis, pitting edema, systemic lupus-like reactions, and leukopenia with bone marrow changes.
Valproic acid serum concentrations should be measured in most patients. Because epilepsy is an episodic disease state, patients do not experience seizures on a continuous basis. Thus, during dosage titration it is difficult to tell if the patient is responding to drug therapy or simply is not experiencing any abnormal central nervous system discharges at that time. Valproic acid serum concentrations are also valuable tools to avoid adverse drug effects. Patients are more likely to accept drug therapy if adverse reactions are held to the absolute minimum.
BASIC CLINICAL PHARMACOKINETIC PARAMETERS
Valproic acid is primarily eliminated by hepatic metabolism (>95%). Hepatic metabolism is via glucuronidation, β-oxidation, and α-hydroxylation. When measured using in vitro techniques, glucuronidation is mediated by UGT1A3, UGT1A4, UGT1A6, UGT1A8, UGT1A9, UGT1A10, UGT2B7, and UGT2B15, with UGT1A6 and UGT2B7 responsible for the majority of the reactions.19–22 Over 10 metabolites have been identified for valproic acid, and the 4-en-valproic acid metabolite may be associated with the drug’s propensity to cause hepatotoxicity. About 1%-5% of a valproic acid dose is recovered in the urine as unchanged drug. Valproic acid follows nonlinear pharmacokinetics due to saturable, or concentration-dependent, plasma protein binding. This is the type of nonlinear pharmacokinetics that occurs when the number of drug molecules overwhelms or saturates albumin’s ability to bind the drug in the plasma. When this occurs, total steady-state drug serum concentrations increase in a disproportionate manner after a dosage increase, but unbound steady-state drug serum concentrations increase in a proportional fashion (see Figure 12-2). Valproic acid is eliminated almost completely by hepatic metabolism, and it is a low-hepatic extraction ratio drug. In this case the hepatic clearance rate is described by the classic relationship that is used to describe hepatic clearance: ClH = [LBF • (fBCl′int)]/(LBF + fBCl′int), where LBF is liver blood flow, fB is the unbound fraction of drug in the blood, and Cl′int is the intrinsic ability of the enzyme system to metabolize the drug. Since valproic acid has a low hepatic extraction ratio, this expression for hepatic clearance simplifies to ClH = fBCl′int.
The clinical implication of concentration-dependent plasma protein–binding pharmacokinetics is that the clearance of valproic acid is not a constant as it is with linear pharmacokinetics, but is concentration or dose dependent. As the dose or concentration of valproic acid increases, the clearance rate (Cl) increases because more unbound drug is available to hepatic enzymes for metabolism: ↑ClH = ↑fBCl′int. This is the reason total steady-state concentrations increase disproportionately after a valproic acid dosage increase: ↑Css = [F(⇑D/τ)]/↑ClH, where F is valproic acid bioavailability, D is valproic acid dose, τ is the dosage interval, and ClH is hepatic clearance. When valproic acid dose is increased, the unbound fraction increases and causes an increase in hepatic clearance. Because both dose and hepatic clearance simultaneously increase, total valproic acid concentrations increase, but by a smaller than expected amount. For example, valproic acid follows concentration-dependent plasma protein–binding pharmacokinetics with average unbound fractions of 5% in the lower end of the therapeutic range (50 μg/mL) and 10% in the upper end of the therapeutic range (100 μg/mL). When the dose is increased and steady-state concentration of valproic acid increases from 50 μg/mL to 100 μg/mL, the unbound fraction increases by a factor of 2 from 5% to 10% and hepatic clearance of total drug will double within the therapeutic range: 2ClH = 2fBCl′int. Unfortunately, there is so much interpatient variability in concentration-dependent plasma protein–binding parameters for valproic acid that predicting changes in unbound fraction and hepatic clearance is extremely difficult. However, since unbound steady-state concentrations are only influenced by intrinsic clearance, unbound concentrations increase in a proportional amount to dose: Css,u = [F(D/τ)]/Cl′int.
Valproic acid volume of distribution (V = 0.15-0.2 L/kg) is also effected by concentration-dependent plasma protein binding and is determined by the physiological volume of blood (VB) and tissues (VT) as well as the unbound fraction of drug in the blood (fB) and tissues (fT): V = VB+ (fB/fT)VT. As valproic acid concentrations increase, unbound fraction of drug in the blood increases which causes an increase in the volume of distribution for the drug: ↑V = VB + (↑fB/fT)VT. Half-life (t1/2) is related to clearance and volume of distribution using the same equation as for linear pharmacokinetics: t1/2 = (0.693 • V)/Cl. However, since clearance and volume of distribution are a function of dose- or concentration-dependent plasma protein binding for valproic acid, half-life also changes with drug dosage or concentration changes. As doses or concentrations increase for a drug that follows concentration-dependent plasma protein–binding pharmacokinetics, clearance and volume of distribution simultaneously increase, and half-life changes are variable depending on the relative changes in clearance and volume of distribution: ↔t1/2 = (0.693 • ↑V)/↑Cl. Using the average clearance and the volume of distribution for an adult (V = 0.15 L/kg, Cl = 10 mL/h/kg or 0.010 L/h/kg), half-life remains at 10 h [t1/2 = (0.693 • V)/Cl] = [0.693 • 0.15 L/kg]/0.010 L/h/kg = 10 h. Clearance and volume of distribution increase to 0.30 L/kg and 0.020 L/h/kg, respectively, due to decreased protein binding: t1/2 = [0.693 • 0.30 L]/0.020 L/h/kg = 10 h as valproic acid serum concentrations increase from 50 μg/mL to 100 μg/mL. The clinical implication of this finding is that the time to steady-state (3-5 t1/2) is variable as the dose or concentration is increased for valproic acid. On average, valproic acid half-life is 12-18 hours in adult patients with total concentrations within the therapeutic range.
Valproic acid is available as three different entities, and all of them are prescribed as valproic acid equivalents: valproic acid, sodium valproate (the sodium salt of valproic acid), and divalproex sodium (a stable coordination compound consisting of a 1:1 ratio of valproic acid and sodium valproate). For parenteral use, valproic acid is available as a 100 mg/mL solution. When given intravenously, it should be diluted in at least 50 mL of intravenous solution, and given over 1 hour (injection rates should not exceed 20 mg/min). For oral use, a syrup (50 mg/mL), soft capsule (250 mg), enteric coated capsules (125, 250, and 500 mg), extended-release tablets (250 mg and 500 mg) and sprinkle capsule (125 mg, used to sprinkle into foods) are available. The enteric-coated capsules are not extended-release products, but only delay the absorption of drug after ingestion. As a result, there are less gastrointestinal side effects with the enteric-coated product.
The oral bioavailability of valproic acid is very good for all dosage forms and ranges from 90% for the extended-release tablets to 100% for the other oral dosage forms. Extended-release tablets produce an AUC that is about 10% less than other oral dosage forms, and drug serum concentrations should be measured for patients converted between extended-release and other oral dosage forms.23,24 If a patient is stabilized on an oral valproic acid product, and it is necessary to switch the patient to intravenous drug, the same total daily dose of injectable valproic acid can be given to the individual. Usually, valproic acid doses are not fine tuned to the point of directly accounting for the difference in valproic acid bioavailability. Rather, clinicians are aware that when valproic acid dosage forms are changed, the serum concentration versus time profile may change. Because of this, most individuals recheck valproic acid steady-state serum concentrations after a dosage form change is instituted.
The typical maintenance dose for valproic acid is 15 mg/kg/d resulting in 1000 mg or 500 mg twice daily for most adult patients. However, because age and coadministration of other antiepileptic drugs that are enzyme inducers (eg, carbamazepine, phenytoin, phenobarbital) effect valproic acid pharmacokinetics, many clinicians recommend the administration of 7.5 mg/kg/d for adults or 10 mg/kg/d for children under 12 years of age receiving monotherapy and 15 mg/kg/d for adults or 20 mg/kg/d for children under 12 years old receiving other drugs that are enzyme inducers.25
EFFECTS OF DISEASE STATES AND CONDITIONS ON PHARMACOKINETICS AND DOSING
For valproic acid, oral clearance (Cl/F) is 7-12 mL/h/kg and half-life is 12-18 hours for adults.26 In children 6-12 years old, oral clearance and half-life equal 10-20 mL/h/kg and 6-8 hours, respectively.27 Clearance rates can be higher and half-lives shorter in patients receiving other hepatic drug-metabolizing enzyme inducers (phenytoin, phenobarbital, carbamazepine). For adults receiving other antiepileptic drugs that are enzyme inducers, valproic acid clearance for adults is 15-18 mL/h/kg and half-lives range from 4 to 12 hours. Similarly, if children receive therapy with other antiepileptic drugs that are enzyme inducers, clearance is 20-30 mL/h/kg and half-life is 4-6 h.28,29 Valproic acid volume of distribution (V/F) is 0.15-0.2 L/kg.26,30
Patients with liver cirrhosis or acute hepatitis have reduced valproic acid clearance because of destruction of liver parenchyma.31 This loss of functional hepatic cells reduces the amount of enzymes available to metabolize the drug and decreases clearance. Valproic acid clearance in patients with liver disease is 3-4 mL/h/kg. The volume of distribution may be larger because of reduced plasma protein binding (free fraction ≈ 29%). Protein binding may be reduced and unbound fraction maybe increased due to hypoalbuminemia and/or hyperbilirubinemia (especially albumin ≤3 g/dL and/or total bilirubin ≥2 mg/dL). Average half-life for valproic acid in patients with liver disease is 25 hours. However, the effects that liver disease has on valproic acid pharmacokinetics are highly variable and difficult to accurately predict. It is possible for a patient with liver disease to have relatively normal or grossly abnormal valproic acid clearance and volume of distribution. For example, a liver disease patient who has relatively normal albumin and bilirubin concentrations can have a normal volume of distribution for valproic acid. An index of liver dysfunction can be gained by applying the Child-Pugh clinical classification system to the patient (Table 12-3).32 Child-Pugh scores are completely discussed in Chapter 3 (Drug Dosing in Special Populations: Renal and Hepatic Disease, Dialysis, Heart Failure, Obesity, and Drug Interactions), but will be briefly discussed here. The Child-Pugh score consists of five laboratory tests or clinical symptoms: serum albumin, total bilirubin, prothrombin time, ascites, and hepatic encephalopathy. Each of these areas is given a score of 1 (normal) to 3 (severely abnormal; see Table 12-3), and the scores for the five areas are summed. The Child-Pugh score for a patient with normal liver function is 5 while the score for a patient with grossly abnormal serum albumin, total bilirubin, and prothrombin time values in addition to severe ascites and hepatic encephalopathy is 15. A Child-Pugh score greater than 8 is grounds for a decrease of 25%-50% in the initial daily drug dose for valproic acid. As in any patient with or without liver dysfunction, initial doses are meant as starting points for dosage titration based on patient response and avoidance of adverse effects. Since the drug has been associated with hepatic damage, valproic acid therapy should be avoided in patients with liver disease whenever possible. Valproic acid serum concentrations and the presence of adverse drug effects should be monitored frequently in patients with liver cirrhosis.
Elderly patients have lower valproic acid oral clearance rates and higher unbound fractions than younger adults, so lower initial doses may be used in older individuals.14 For patients between 65 and 99 years old, there does not appear to be a further decline in valproic acid clearance as age advances.33 During the third trimester of pregnancy, oral clearance of valproic acid may decrease and require dosage adjustment.34 Valproic acid serum concentrations exhibit some diurnal variation in patients, so the time that steady-state serum concentrations are obtained should be noted when comparing multiple values.14,35 Doses of valproic acid do not require adjustment for patients with renal failure, and the drug is not removed by dialysis.36 Breast milk concentrations of valproic acid are about 10% of concurrent serum concentrations.
DRUG INTERACTIONS
Valproic acid is a potent inhibitor of hepatic drug-metabolizing enzyme systems and glucuronidation.37–40 Other antiepileptic drugs that have their clearance rates decreased and steady-state concentrations increased by valproic acid–related enzyme inhibition include clonazepam, carbamazepine, phenytoin, primidone, lamotrigine, and ethosuximide. Valproic acid therapy also decreases the clearance and increases steady-state concentrations of other drugs including zidovudine, amitriptyline, and nortriptyline. As a general rule, when valproic acid is added to a patient’s drug regimen, an adverse effect due to one of the other drugs must be considered as a possible drug interaction with valproic acid.
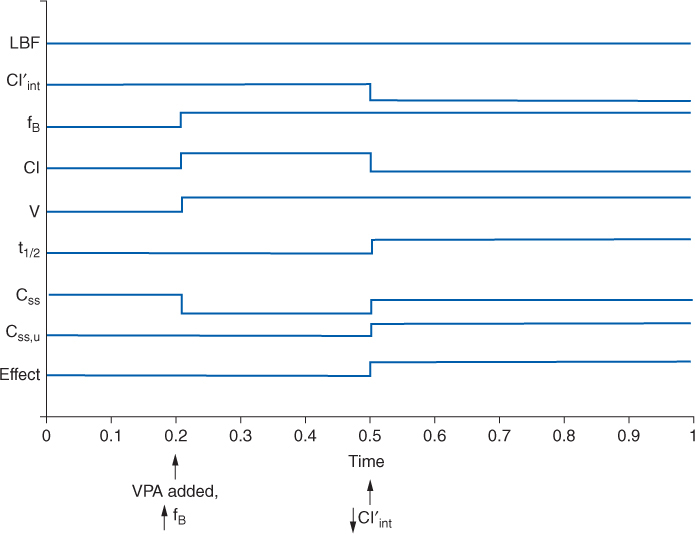
Additionally, other drugs can affect valproic acid clearance and steady-state serum concentrations.40 Phenytoin, lamotrigine, rifampin, and carbamazepine can increase valproic acid clearance and decrease valproic acid steady-state serum concentrations. Cimetidine, chlorpromazine, and felbamate are examples of drugs that decrease valproic acid clearance and increase valproic acid steady-state concentrations. Carbapenem antibiotics decrease valproic acid concentrations to the extent that of loss of seizure control has occurred in some epileptic patients. Because valproic acid is highly protein bound, plasma protein–binding drug interactions can occur with other drugs that are highly bound to albumin.40 Aspirin, warfarin, and phenytoin all have plasma protein–binding drug interactions with valproic acid, and these drugs have higher unbound fractions when given concurrently with valproic acid. The drug interaction between valproic acid and phenytoin deserves special examination because of its complexity and because these two agents are regularly used together for the treatment of seizures.41–44 The drug interaction involves the plasma protein–binding displacement and intrinsic clearance inhibition of phenytoin by valproic acid. What makes this interaction so difficult to detect and understand is that these two changes do not occur simultaneously, so the impression left by the drug interaction depends on when in time it is observed in a patient. For example, a patient is stabilized on phenytoin therapy (Figure 12-3), but because adequate control of seizures has not been attained, valproic acid is added to the regimen. As valproic acid concentrations accumulate, the first interaction observed is phenytoin plasma protein binding as the two drugs compete for binding sites on albumin. The result of this portion of the drug interaction is an increase in phenytoin-unbound fraction and a decrease in phenytoin total serum concentration, but the unbound phenytoin serum concentration remains the same. As valproic acid serum concentrations achieve steady-state conditions, the higher concentrations of the drug bathe the hepatic microsomal enzyme system and inhibit the intrinsic clearance of phenytoin. This portion of the interaction decreases intrinsic clearance and hepatic clearance for phenytoin, so both unbound and total phenytoin concentrations increase. When phenytoin concentrations finally equilibrate and reach steady-state under the new plasma protein binding and intrinsic clearance conditions imposed by concurrent valproic acid therapy, the total concentration of phenytoin is oftentimes at about the same level as before the drug interaction occurred, but unbound phenytoin concentrations are much higher. If only total phenytoin concentrations are measured at this point in time, clinicians will be under the impression that total concentrations did not change and no drug interaction occurred. However, if unbound phenytoin concentrations are simultaneously measured, it will be found that these concentrations have risen and that the phenytoin-unbound fraction is twice or more (≥20%) of the baseline amount. In this situation, the patient may have unbound phenytoin concentrations that are toxic and a decrease in phenytoin dosage may be in order.
FIGURE 12-3 Schematic representation of the effect on physiologic (LBF = liver blood flow, Cl′int = intrinsic or unbound clearance, fB = unbound fraction of drug in blood/plasma), pharmacokinetic (Cl = clearance; V = volume of distribution; t1/2 = half-life; Css = total steady-state drug concentration; Css, u = unbound steady-state drug concentration), and pharmacodynamic (Effect = pharmacodynamic effect) parameters that occur when initiating valproic acid (VPA) treatment in an individual stabilized on phenytoin therapy. Initially, valproic acid decreases phenytoin plasma-protein binding via competitive displacement for binding sites on albumin (arrow denotes ↑fB). As valproic acid concentrations increase, the hepatic enzyme inhibition component of the drug interaction comes into play (arrow denotes ↓ Cl′int). The net result is total phenytoin concentrations are largely unchanged from baseline, but unbound phenytoin concentrations and pharmacologic effect increase.
INITIAL DOSAGE DETERMINATION METHODS
Several methods to initiate valproic acid therapy are available. The Pharmacokinetic Dosing method is the most flexible of the techniques. It allows individualized target serum concentrations to be chosen for a patient, and each pharmacokinetic parameter can be customized to reflect specific disease states and conditions present in the patient. Literature-based Recommended dosing is a very commonly used method to prescribe initial doses of valproic acid. Doses are based on those that commonly produce steady-state concentrations in the lower end of the therapeutic range, although there is a wide variation in the actual concentrations for a specific patient.
Pharmacokinetic Dosing Method
The goal of initial dosing of valproic acid is to compute the best dose possible for the patient given their set of disease states and conditions that influence valproic acid pharmacokinetics and the epileptic disorder being treated. In order to do this, pharmacokinetic parameters for the patient will be estimated using average parameters measured in other patients with similar disease state and condition profiles.
Clearance Estimate
Valproic acid is predominately metabolized by liver. Unfortunately, there is no good way to estimate the elimination characteristics of liver-metabolized drugs using an endogenous marker of liver function in the same manner that serum creatinine and estimated creatinine clearance are used to estimate the elimination of agents that are renally eliminated. Because of this, a patient is categorized according to the disease states and conditions that are known to change valproic acid clearance, and the clearance previously measured in these studies is used as an estimate of the current patient’s clearance. For example, for a 70-kg adult patient with liver cirrhosis or acute hepatitis, valproic acid clearance would be assumed to equal 3-4 mL/h/kg: 70 kg • 3.5 mL/h/kg = 245 mL/h or 0.245 L/h. To produce the most conservative valproic acid doses in patients with multiple concurrent disease states or conditions that affect valproic acid pharmacokinetics, the disease state or condition with the smallest clearance should be used to compute doses. This approach will avoid accidental overdosage as much as currently possible.
Volume of Distribution Estimate
Valproic acid volume of distribution is assumed to equal 0.15 L/kg for adults and 0.2 L/kg for children under 12 years of age. Thus, for an 80-kg adult patient, the estimated valproic volume of distribution would be 12 L: V = 0.15 L/kg • 80 kg = 12 L. Patients with cirrhosis or renal failure may have larger volumes of distribution due to decreased plasma protein binding.
Half-Life and Elimination Rate Constant Estimate
Once the correct clearance and volume of distribution estimates are identified for the patient, they can be converted into the valproic acid half-life (t1/2) and elimination rate constant (k) estimates using the following equations: t1/2 = (0.693 • V)/Cl, k = 0.693/t1/2 = Cl/V.
Selection of Appropriate Pharmacokinetic Model and Equations
When given by intravenous injection or orally, valproic acid follows a one-compartment pharmacokinetic model. When oral therapy is required, valproic acid has good bioavailability (F = 1), and every 8-12 hour dosing provides a relatively smooth serum concentration-time curve that emulates an intravenous infusion. Because of this, a very simple pharmacokinetic equation that computes the average valproic acid steady-state serum concentration (Css in μg/mL = mg/L) is widely used and allows maintenance dosage calculation: Css = [F(D/τ)]/Cl or D = (Css • Cl • τ)/F, where F is the bioavailability fraction for the oral dosage form (F = 1 for oral rapid-release products, F = 0.9 for oral extended-release tablets), D is the dose of valproic acid in mg, and τ is the dosage interval in hours. Cl is valproic acid clearance in L/h. When intravenous therapy is required, the same pharmacokinetic equation is widely used: Css = (D/τ)/Cl or D = Css • Cl • τ, where D is the dose of valproic acid in mg, and τ is the dosage interval in hours. Cl is valproic acid clearance in L/h.
The equation used to calculate an intravenous loading dose (LD in mg) is based on a simple one-compartment model: LD = Css • V, where Css is the desired valproic acid steady-state concentration in μg/mL which is equivalent to mg/L, and V is the valproic acid volume of distribution. Intravenous valproic acid doses should be infusions over at least 60 minutes (≤20 mg/minute).
Literature-Based Recommended Dosing
Because of the large amount of variability in valproic acid pharmacokinetics, even when concurrent disease states and conditions are identified, most clinicians believe that the use of standard valproic acid doses for various situations is warranted. The original computation of these doses were based on the Pharmacokinetic Dosing methods and subsequently modified based on clinical experience. In general, the expected valproic acid steady-state serum concentrations used to compute these doses was 50 μg/mL. Usual initial maintenance doses for pediatric patients are 10 mg/kg/d if the child is not taking a hepatic enzyme inducer (phenytoin, phenobarbital, carbamazepine, rifampin) or 20 mg/kg/d if the child is taking a hepatic enzyme inducer. For adults, initial maintenance doses are 7.5 mg/kg/d if the patient is not taking hepatic enzyme inducers or 15 mg/kg/d if a hepatic enzyme inducer is concurrently administered. Two or three divided daily doses are initially used for these total doses. To avoid gastrointestinal side effects, doses over 1500 mg given at one time should be avoided. Dosage increases of 5-10 mg/kg/d are made every 1-2 weeks depending on response and adverse effects. Most adults will require 1500-3000 mg/d of valproic acid. If the patient has significant hepatic dysfunction (Child-Pugh score ≥8), maintenance doses prescribed using this method should be decreased by 25%-50% depending on how aggressive therapy is required to be for the individual.
To illustrate the similarities and differences between this method of dosage calculation and the Pharmacokinetic Dosing method, the same examples used in the previous section will be used.