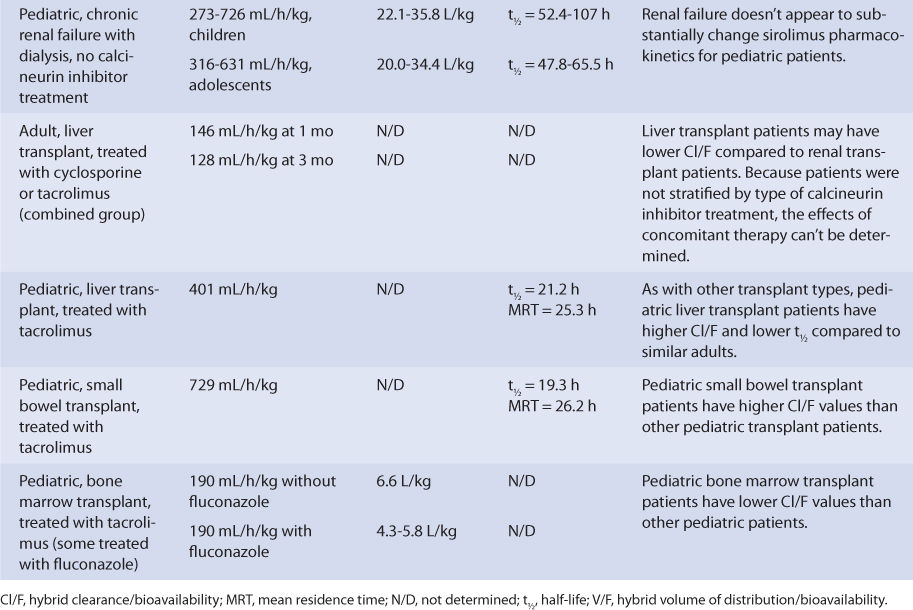
Adult liver transplant patients taking a calcineurin inhibitor (patients not stratified by type of calcineurin inhibitor) had a sirolimus Cl/F of 146 mL/h/kg after 1 month of therapy, while pediatric liver transplant patients being treated with tacrolimus had a sirolimus Cl/F equal to 401 mL/h/kg and a half-life equal to 21.2 hours. The posttransplant time was 6-144 months for the adults, and the median posttransplant time equaled 8.3 months for the children. If administered immediately after liver transplantation, sirolimus metabolism may be depressed until the graft begins functioning in a stable manner.
Pediatric small bowel transplant patients receiving therapy with tacrolimus had a Cl/F of 729 mL/h/kg, a half-life of 19.3 hours, and an MRT of 26.2 hours. The median posttransplant time was 25.4 months for these patients when sirolimus pharmacokinetic parameters were determined, so the small bowel graft was likely stable with consistent sirolimus oral absorption. For small bowel transplant patients, if the graft is not functioning optimally, malabsorption of sirolimus can cause a decrease in bioavailability, which would make Cl/F increase.
Pediatric bone marrow transplant patients treated with tacrolimus had a Cl/F equal to 190 mL/min/kg and a V/F equal to 4.3-6.6 L/kg. Some of these patients were taking fluconazole concurrently, which can inhibit sirolimus hepatic metabolism.20,32
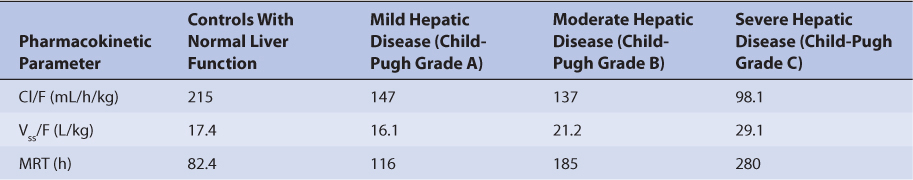
Because the drug is primarily eliminated by hepatic metabolism, average clearance is lower in adult patients with liver dysfunction.45,46 Using the Child-Pugh rating system (Table 20-2), patients with mild liver disease had a sirolimus Cl/F of 147 mL/h/kg, patients with moderate liver disease had a sirolimus Cl/F equal to 137 mL/h/kg, and patients with severe liver disease had a sirolimus Cl/F of 98.1 mL/h/kg (Table 20-3). Also, mean volume of distribution was larger, and mean residence time was prolonged and variable in this patient population. Immediately after liver transplantation, sirolimus metabolism may be depressed until the graft begins functioning in a stable manner. Additionally, patients with transient liver dysfunction, regardless of transplantation type, will have decreased sirolimus clearance and increased half-life values. Because only a small amount of sirolimus is eliminated in the urine, it is unlikely that renal failure substantially alters its clearance.8 Since sirolimus is a relatively large molecule (molecular weight = 914.2 Da), very lipophilic, and highly bound in the blood, it is probable that it is not highly removed by various renal dialysis techniques. Sirolimus pharmacokinetics in renal failure patients and patients undergoing dialysis requires further investigation to provide a definitive answer for the best dosing of the drug in these groups.
TABLE 20-2 Child-Pugh Scores for Patients With Liver Disease47
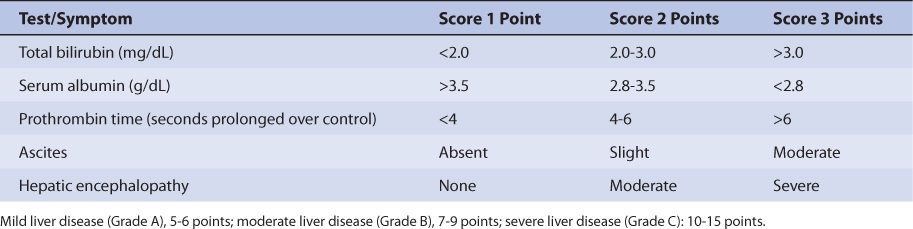
TABLE 20-3 Sirolimus Pharmacokinetics in Patients With Hepatic Dysfunction
DRUG INTERACTIONS
Compared with cyclosporine, sirolimus drug interactions are not as well documented, and many drug interactions that are reported with calcineurin inhibitors are assumed to also occur with sirolimus.8,20,32 Drug interactions with sirolimus involve inhibition or induction of its metabolism by other drugs.32 Sirolimus is metabolized by CYP3A4 and is a substrate for p-glycoprotein, so the potential for many pharmacokinetic drug interactions exists with agents that inhibit these pathways or are also cleared by these mechanisms. Because both of these drug elimination systems also exist in the gastrointestinal tract, inhibition drug interactions may also enhance sirolimus oral bioavailability by diminishing the intestinal and hepatic first-pass effects. Drugs that may inhibit sirolimus metabolism include the calcium channel blockers (verapamil, diltiazem, nicardipine), azole antifungals (clotrimazole, fluconazole, itraconazole, ketoconazole, posaconazole, voriconazole), macrolide antibiotics (erythromycin, clarithromycin, azithromycin, troleandomycin), antivirals (atazanavir, darunavir, indinavir, nelfinavir, ritonavir, saquinavir, telaprevir, delavirdine), antiarrhythmics (amiodarone, dronedarone), psychotropic agents (fluvoxamine, nefazodone) as well as other compounds (quinupristin, telithromycin, aprepitant, berberine, conivaptan, grapefruit juice). Inducing agents include other antibiotics (rifampin, rifabutin, rifapentine), other antivirals (efavirenz, nevirapine), anticonvulsants (phenytoin, carbamazepine, oxcarbazepine, phenobarbital, primidone), barbiturates, bosentan, and St. John’s Wort. Because of the large number of potentially interacting agents, and the critical nature of the drugs involved in the treatment of transplant patients, complete avoidance of drug interactions with sirolimus is not possible. Thus, most drug interactions with sirolimus are managed using appropriate sirolimus dosage modification with sirolimus concentration monitoring as a guide.
Sirolimus is frequently administered with calcineurin inhibitors, and tacrolimus coadministration does not appear to have a significant effect on sirolimus pharmacokinetics.37,48 However, cyclosporine is a potent inhibitor of sirolimus metabolism.8,20,32 This effect can be mitigated by administering sirolimus about 4 hours after the cyclosporine dose is given, but steady-state concentrations of both immunosuppressants should be monitored with doses adjusted appropriately.38,49 For patients receiving concomitant cyclosporine and sirolimus treatment, sirolimus steady-state concentrations may fall if cyclosporine therapy is discontinued. If cyclosporine treatment is halted, sirolimus concentrations should be measured, and the sirolimus dose increased if levels decline below therapeutic amounts.
INITIAL DOSAGE DETERMINATION METHODS
Several methods to initiate sirolimus therapy are available. The Pharmacokinetic Dosing method is the most flexible of the techniques. It allows individualized target serum concentrations to be chosen for a patient, and each pharmacokinetic parameter can be customized to reflect specific disease states and conditions present in the patient. Literature-based recommended dosing is the most common method used to prescribe initial doses of sirolimus. Doses are based on those that commonly produce steady-state concentrations in the therapeutic range, although there is a wide variation in the actual concentrations for a specific patient.
Pharmacokinetic Dosing Method
The goal of initial dosing of sirolimus is to compute the best dose possible for the patient in order to prevent graft rejection or graft-versus-host disease given their set of disease states and conditions that influence sirolimus pharmacokinetics, while avoiding adverse drug reactions. In order to do this, pharmacokinetic parameters for the patient will be estimated using average parameters measured in other patients with similar disease state and condition profiles.
Clearance Estimate
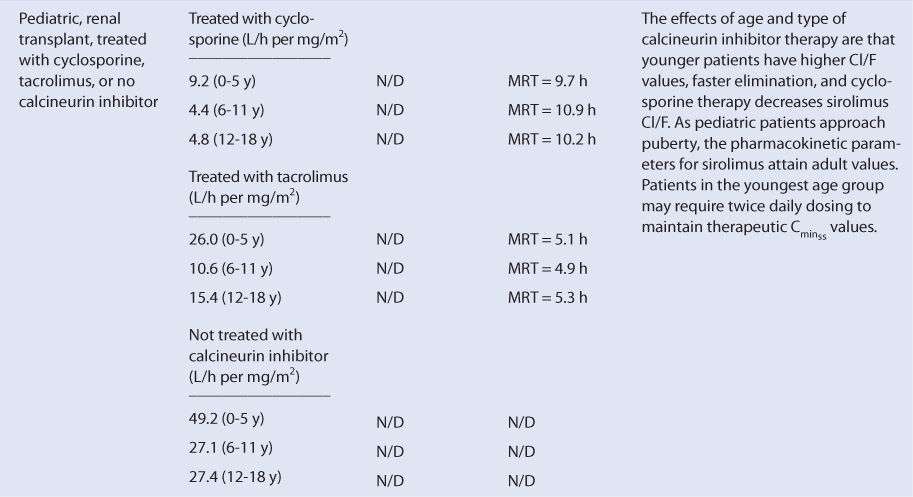
Sirolimus is almost completely metabolized by the liver. Unfortunately, there is no good way to estimate the elimination characteristics of liver-metabolized drugs using an endogenous marker of liver function in the same fashion that serum creatinine and estimated creatinine clearance are used to estimate the elimination of agents that are renally eliminated. Because of this, a patient is categorized according to the disease states and conditions that are known to change sirolimus clearance, and the clearance previously measured in these studies is used as an estimate of the current patient’s clearance rate (see Table 20-1). For example, an adult transplant patient with normal liver function treated concomitantly with cyclosporine would be assigned a sirolimus Cl/F value equal to 240 mL/h/kg and a V/F value of 10 L/kg, while a pediatric transplant patient in the child age group with the same profile would be assumed to have a sirolimus Cl/F of 485 mL/h/kg (V/F value not determined).
Selection of Appropriate Pharmacokinetic Model and Equations
When oral therapy is chosen for agents with a large first-pass metabolism, the drug is often erratically absorbed with variable absorption rates. Because of the complex absorption profile and the fact that the drug is usually administered once daily for adults and twice daily for younger children, a very simple pharmacokinetic equation that calculates the average sirolimus steady-state concentration (Css in ng/mL = μg/L) is widely used and allows maintenance dose computation: Css = (D/τ)/(Cl/F) or D = Css(Cl/F)τ, where D is the dose of sirolimus in mg, Cl/F is sirolimus hybrid clearance/bioavailability parameter in L/h, and τ is the dosage interval in hours. If a loading dose (LD) of the drug is to be given, the equation that computes it is LD = Css(V/F), where V/F is the sirolimus hybrid volume of distribution/bioavailability parameter in L.
Steady-State Concentration Selection
The generally accepted therapeutic range for sirolimus in the blood is 5-15 ng/mL. For renal transplant patients with a low or moderate immunologic risk, recommended therapeutic steady-state sirolimus (typically 10-15 ng/mL) and cyclosporine concentrations should be maintained. If an adequate response has been attained during that time frame, the cyclosporine dose is tapered off over 4-8 weeks, and the sirolimus dose is adjusted to maintain therapeutic steady-state concentrations. Because cyclosporine inhibits sirolimus metabolism, the sirolimus dose usually needs to be increased while cyclosporine doses are decreased. For this clinical scenario, the recommended sirolimus steady-state concentrations after cyclosporine withdrawal are 16-24 ng/mL for the first year after transplantation and 12-20 ng/mL thereafter.4,8 For renal transplant patients with a high immunologic risk (defined as Black transplant recipients, past transplant recipients with a failed kidney graft due to an immunologic etiology, or patients with lab results indicating high panel-reactive antibodies), sirolimus therapy is initiated with cyclosporine and corticosteroids for 1 year following transplantation. Therapeutic steady-state sirolimus (typically 10-15 ng/mL) and cyclosporine concentrations should be maintained. If an adequate response has been attained during that time frame, adjustments to the dosage regimens for the individual medications are based on the clinical response of the patient and may include reduction of the cyclosporine dose.4,8 If tacrolimus is prescribed as the calcineurin inhibitor, the same dosage approaches can be used.
More important than these general guidelines are the specific requirements for each graft type as defined by the transplant center where the surgery was conducted. Clinicians should become familiar with the sirolimus protocols used at the various institutions at which they practice. During the early posttransplantation phase, sirolimus concentrations are measured at least every 5-7 days in most patients during dosage initiation or after a dosage adjustment in solid organ transplant patients or graft-versus-host disease in hematopoietic stem cell transplant patients. After discharge from the hospital, sirolimus concentrations continue to be obtained at most clinic visits.
Literature-Based Recommended Dosing
Because of the large amount of variability in sirolimus pharmacokinetics, even when concurrent disease states and conditions are identified, many clinicians believe that the use of standard sirolimus doses for various situations is warranted. Indeed, most transplant centers use doses that are determined using a sirolimus dosage protocol. The original computations of these doses were based on the Pharmacokinetic Dosing method described in the previous section, and subsequently modified based on clinical experience. In general, the expected sirolimus steady-state concentration used to compute these doses is dependent upon the type of transplanted tissue and the posttransplantation time line. Because the steady-state-to-single dose accumulation ratio for sirolimus is ~3, recommended loading doses are usually three times the prescribed maintenance dose.8 Many transplant centers omit loading doses and commence dosing only with the maintenance dose.4
The implementation of these strategies is best established for renal transplant patients 13 years old and above. The dosage for sirolimus is stratified by the patient’s immunologic risk. For patients with a low or moderate immunologic risk, sirolimus therapy is initiated using a 6-mg loading dose, followed by 2 mg/d with concomitant cyclosporine and corticosteroids therapy for 2-4 months. Therapeutic steady-state sirolimus (typically 10-15 ng/mL) and cyclosporine concentrations should be maintained. If an adequate response has been attained during that time frame, the cyclosporine dose is tapered off over 4-8 weeks, and the sirolimus dose is adjusted to maintain therapeutic steady-state concentrations. Because cyclosporine inhibits sirolimus metabolism, the sirolimus dose usually needs to be increased while cyclosporine doses are decreased. For this clinical scenario, the recommended sirolimus steady-state concentrations after cyclosporine withdrawal are 16-24 ng/mL for the first year after transplantation and 12-20 ng/mL thereafter.4,8
For patients with a high immunologic risk (defined as Black transplant recipients, past transplant recipients with a failed kidney graft due to an immunologic etiology, or patients with lab results indicating high panel-reactive antibodies), sirolimus therapy is initiated using a loading dose up to 15 mg, followed by 5 mg/d with concurrent cyclosporine and corticosteroids treatment for 1 year following transplantation. Therapeutic steady-state sirolimus (typically 10-15 ng/mL) and cyclosporine concentrations should be maintained. If an adequate response has been attained during that time frame, adjustments to the dosage regimens for the individual medications are based on the clinical response of the patient and may include reduction of the cyclosporine dose.4,8 If tacrolimus is prescribed as the calcineurin inhibitor, the same dosage approaches can be used.
Sirolimus doses for adolescents 13 years old or older with low body weight (<40 kg) undergoing renal transplantation should be computed using body surface area. The recommended amounts are a loading dose of 3 mg/m2 and an initial maintenance equal to 1 mg/m2/d. Body surface area (BSA in m2) can be estimated using the following formula50: BSA = 0.007184 • W0.425 • H0.725, where W is weight in kg and H is height in cm.
USE OF SIROLIMUS CONCENTRATIONS TO ALTER DOSES
Because of the large amount of pharmacokinetic variability among patients, it is likely that doses computed using patient population characteristics will not always produce sirolimus concentrations that are expected or desirable. Because of pharmacokinetic variability, the narrow therapeutic index of sirolimus, and the severity of sirolimus adverse side effects, measurement of sirolimus concentrations is mandatory for patients to ensure that therapeutic, nontoxic levels are present. In addition to sirolimus concentrations, important patient parameters (transplanted organ function tests or biopsies, clinical signs and symptoms of graft rejection or graft-versus-host disease, potential sirolimus side effects, etc) should be followed to confirm that the patient is responding to treatment and not developing adverse drug reactions.
For most patients, predose steady-state trough sirolimus concentrations are typically measured. Since alternate methods to monitor cyclosporine concentrations have met with some success, investigators have begun suggesting similar methods for sirolimus. Of these methods, estimation of sirolimus area under the concentration-time curve (AUC) using several measured steady-state concentrations is the one that is used in some transplant centers.
When sirolimus concentrations are measured in patients and a dosage change is necessary, clinicians should seek to use the simplest, most straightforward method available to determine a dose that will provide safe and effective treatment. In most cases, a simple dosage ratio can be used to change sirolimus doses assuming the drug follows linear pharmacokinetics. Sometimes, it is useful to compute sirolimus pharmacokinetic constants for a patient and base dosage adjustments on these. In this case, it may be possible to calculate and use pharmacokinetic parameters to alter the sirolimus dose. Another approach involves measuring several postdose steady-state sirolimus concentrations to estimate the area under the concentration-time curve (AUC) and adjusting the sirolimus dose to attain a target AUC. Finally, computerized methods that incorporate expected population pharmacokinetic characteristics (Bayesian pharmacokinetic computer programs) can be used in difficult cases where concentrations are obtained at suboptimal times or the patient was not at steady-state when concentrations were measured.
Linear Pharmacokinetics Method
Assuming sirolimus follows linear, dose-proportional pharmacokinetics, steady-state concentrations change in proportion to dose according to the following equation:1,4,9 Dnew/Css,new = Dold/Css,old or Dnew = (Css,new/Css,old)Dold, where D is the dose, Css is the steady-state concentration, old indicates the dose that produced the steady-state concentration that the patient is currently receiving, and new denotes the dose necessary to produce the desired steady-state concentration. The advantages of this method are that it is quick and simple. The disadvantage is steady-state concentrations are required.
Pharmacokinetic Parameter Method
The Pharmacokinetic Parameter method of adjusting drug doses was among the first techniques available to change doses using drug concentrations. It allows the computation of an individual’s own, unique pharmacokinetic constants and uses those to calculate a dose that achieves desired sirolimus concentrations. The Pharmacokinetic Parameter method requires that steady-state has been achieved and uses only a steady-state sirolimus concentration. Sirolimus clearance can be measured using a single steady-state sirolimus concentration and the following formula for orally administered drug: Cl/F = (D/τ)/Css, where Cl/F is the sirolimus clearance/bioavailability hybrid constant in L/h, τ is the dosage interval in hours, and Css is the sirolimus steady-state concentration in ng/mL which also equals μg/L. Although this method does allow computation of sirolimus Cl/F, it yields exactly the same sirolimus dose as that supplied using linear pharmacokinetics. As a result, most clinicians prefer to directly calculate the new dose using the Simpler Linear Pharmacokinetics method. To demonstrate this point, the patient cases used to illustrate the linear pharmacokinetics method will be used as examples for the Pharmacokinetic Parameter method.












