Fig. 25.1 Botulinum neurotoxin A (BoNT/A) may have various ways in relieving neuropathic pain. However, the central effect is still debated.
Botulinum neurotoxin for pain relief
Botulinum neurotoxin has been used effectively to alleviate pain in certain pain disorders, including migraine, myofascial pain syndrome, plantar fasciitis, arthritis and carpal tunnel syndrome (Jabbari and Machado, 2011). This has extended the field of traditional pain therapy, particularly for the unsatisfactory treatment of neuropathic pain. In fact, BoNT can suppress both nociceptor sensitization and neuropathic pain and has been shown to have a pain-relieving effect in peripheral neuropathic pain of various conditions (Ranoux et al., 2008; Yuan et al., 2009).
Botulinum neurotoxin also has a role in the treatment of ischemic pain in vasospastic disorders (Neumeister et al., 2009).
How botulinum neurotoxin works with neuropathic pain
Abnormal muscle contractions contribute to chronic pain (Gunn, 2001). Botulinum neurotoxin serotypeA (BoNT-A) is well known to have an effect on inhibition of muscle contraction and this may partially explain its effect on chronic pain. It has also been hypothesized that BoNT-A blocks the neurovascular smooth muscle cells, thereby causing vasodilatation (Van Beek et al., 2007).
In preclinical models, BoNT-A was found to effectively block the release of several pain-related neurotransmitters, including norepinephrine, substance P, glutamate and calcitonin gene-related peptide, from afferent nerve terminals (Cui et al., 2004; Aoki, 2005). These pain-related neurotransmitters can stimulate depolarization of C fibers, which are responsible for propagation of chronic pain. The experimental studies showed that interruption of the release of pain-related neurotransmitters could block the peripheral sensitization of pain.
Extracellular ATP is implicated in a considerable number of sensory processes, including pain. Evidence shows that activation of P2X3 purine receptor by ATP leads to a much stronger nociceptive effect in inflamed tissue; BoNT-A has been reported to block these receptors, which are almost exclusively expressed in the sensory neurons (Paukert et al., 2001; Ford et al., 2006).
BoNT-A also blocks the translocation of the capsaicin receptor (vanilloid receptor; a member of the transient receptor potential cation channel subfamily V), a protein that is responsible for perception of peripheral inflammatory pain in peripheral nociceptor terminals (Morenilla-Palao et al., 2004). In a formalin-induced pain study, BoNT-A was found to have an antinociceptive effect, which was associated with the inhibition of glutamate release from the primary afferent terminals (Cui et al., 2004). The excitatory amino acid glutamate is also involved in the induction and maintenance of central sensitization of pain by exerting its postsynaptic effect via NMDA receptors (Petrenko et al., 2003). These findings raise the possibility of potential indirect central effects of BoNT.
In summary, BoNT may act to relieve neuropathic pain via multiple mechanisms (Fig. 25.1). Easing muscle contraction, improving microvascularity, blockade of neurotransmitter release and inhibiting central perception all contribute to the effectiveness of BoNT for neuropathic pain.
Diabetic neuropathic pain
Chronic pain is one of the most common complications of diabetic neuropathy. The prevalence of painful peripheral neuropathy in type 2 diabetes is more than 25% (Davies et al., 2006). There is still no comprehensive understanding of the underlying biologic process responsible for diabetic neuropathic pain. However, it is believed that both neuropathic pain and microangiopathy-related ischemic pain play significant roles.
A randomized double-blind crossover trial demonstrated the pain-relieving effect of BoNT for diabetic foot pain (Yuan et al., 2009). This pilot study included 20 patients with type 2 diabetes who suffered from neuropathic pain in both feet and had been diagnosed with diabetes for at least 3 years. The recruited patients were assessed to confirm the diagnosis of polyneuropathy by (1) physical examination, (2) the neuropathic pain diagnostic questionnaire DN4, and (3) nerve conduction velocity examinations. Participants were randomly assigned to receive either BoNT-A or saline injections as their first treatment and were crossed over to the alternate treatment in the second half of the study. The injections were given at 12 evenly spaced sites across the dorsum of each foot. Each injection comprised either approximately 4 U BoNT-A or 0.9% saline, 0.10 ml, using a 8 mm (5/16-inch) 30-gauge needle. Since the dorsal area of each foot is over 100 cm2 for most people, the average dosage adopted for this trial was below 0.5 U/cm2 (Fig. 25.2). This dosage is much lower than the therapeutic dosage in the study of BoNT-A for trigeminal neuralgia (Piovesan et al., 2005). The visual analog scale (VAS), the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI), and the Short Form-36 (SF-36) quality-of-life questionnaire were given at intervals of 1, 4, 8 and 12 weeks after the initial injection. The second (i.e. crossover) injection was given in the 12th week.
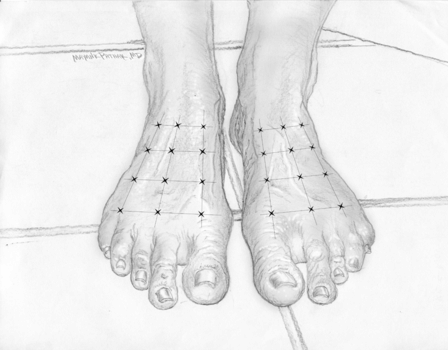
Fig. 25.2 Botulinum neurotoxin for diabetic neuropathic pain: The injections were given at 12 evenly spaced sites across the dorsum of each foot. Each injection comprised approximately 4 U onabotulinumtoxinA/incobotulinumtoxinA. The average dosage adopted for this trial was below 0.5 U/cm2.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


