Fig. 33.1 Anatomical lesions and neurogenic bladder.
A variety of efferent and afferent neural pathways, reflexes and central and peripheral neurotransmitters are involved in urine storage and bladder emptying. Acetylcholine, which interacts with muscarinic receptors on the detrusor muscle, is the predominant peripheral neurotransmitter responsible for bladder contraction. The muscarinic receptor subtype M3 appears to be the most clinically relevant in the human bladder. Acetylcholine interacts with the M3 receptor, initiating a cascade of events that result in contraction of the detrusor muscle. The M2 receptor may also facilitate bladder contraction by reducing intracellular levels of cyclic AMP. Pathological states can alter sensitivity to muscarinic stimulation. For example, bladder outflow obstruction appears to enhance responsiveness to acetylcholine, a phenomenon similar to denervation supersensitivity.
Many classes of drug, particularly anticholinergics, have been studied or proposed for the treatment of symptoms of overactive bladder. All anticholinergic drugs can have bothersome side effects. Although dry mouth is the most common, constipation, gastroesophageal reflux, blurry vision, urinary retention and cognitive side effects can also occur. Since various forms of dementia are routinely treated with cholinesterase inhibitors, the potential for adverse cognitive effects and delirium with antimuscarinic drugs is a particular concern in the older population. Direct injection of BoNT into the detrusor muscle (which inhibits acetylcholine at the presynaptic cholinergic junction but may also have an important role on the afferent pathways of the lower urinary tract [Ikeda et al., 2012]) appears to ameliorate detrusor hyperreflexia in patients with spinal cord injury (Cruz et al., 2011; Herschorn et al., 2011; Ginsberg et al., 2012). It also has therapeutic value in selected patients with severe refractory overactive bladder (Duthie et al., 2011). Injection technique consists of injecting mainly the detrusor and sparing the trigone (Fig. 33.2) using a rigid or a flexible cystoscope. However, a recent randomized study comparing trigone-sparing with trigone-including injections showed better results in the latter group (Manecksha et al., 2012).
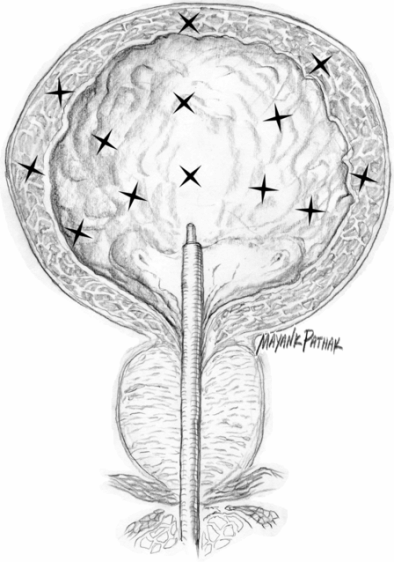
Fig. 33.2 Botulinum neurotoxin A injection into the bladder: detrusor mapping.
Patients have either mild conscious sedation or general anesthesia. Injection doses reported in the literature have varied between 100 and 300 U (mouse units) onabotulinumtoxinA and 500 and 1000 U abobotulinumtoxinA for neurogenic detrusor overactivity. Injection doses have varied between 100 and 300 U onabotulinumtoxinA (diluted from 10 to 30 ml with 0.9% saline) and 300 to 1000 U abobotulinumtoxinA (dilution from 100 UI/ml to 25 UI/ml) for idiopathic detrusor overactivity. Injection sites have varied between 20 and 30 for neurogenic detrusor overactivity and 10 to 30 for idiopathic detrusor overactivity. For each treatment, the recommended dose for abobotulinumtoxinA seems to be 500–750 U and for onabotulinumtoxinA from 100 to 300 U.
Mean duration of improvement has varied between 6 and 9 months with onabotulinumtoxinA and between 5 and 10 months with abobotulinumtoxinA. The continence improvement rate is 86.5% with onabotulinumtoxinA and 86% with abobotulinumtoxinA. No side effects related to the injection itself have been reported with the exception of a risk of urinary tract infections of 7.1–24%; for this reason, some authors recommend the use of antibiotic prophylaxis with a short course of trimethoprim or quinolones. There are occasional reports of general weakness using onabotulinumtoxinA (2 of the 340 treated patients [0.6%]), whereas general weakness has been described in 2.5–5% with abobotulinumtoxinA (255 treated patients). RimabotulinumtoxinB is efficient in treating idiopathic detrusor overactivity, but because of its short duration of effect, it is better used as a secondary treatment in patients who become resistant to BoNT type A products.
Conclusions
Injection with BoNT for overactive bladder appears to be a treatment with high efficacy that meets evidence-based medicine level I criteria (i.e. prospective, randomized controlled clinical trial with masked outcome assessment, in a representative population), with an acceptable safety profile. Repeated injections appear as effective as the first one (Giannantoni et al., 2009; Sahai et al., 2010; Dowson et al., 2012). Additional controlled, double-blind studies are needed and particularly studies in idiopathic detrusor overactivity to further explore this treatment option.
Vulvodynia and chronic pelvic pain
Vulvodynia
Vulvodynia is a chronic disorder in women defined as vulvar discomfort, most often described as burning pain, occurring in the absence of relevant findings or a specific clinically identifiable neurological disorder (Moyal-Barracco and Lynch, 2003) and characterized by provoked or unprovoked vulvar pain of varying intensity without obvious concomitant clinical pathology. Two subtypes of vulvodynia are recognized: generalized and localized. The latter is currently referred to as vestibulodynia or vestibulitis.
In addition to vulvar pain, there is typically burning and, less often, itching. Onset is usually abrupt and the typical patient is between 20 and 45 years of age. Vulvodynia has been shown to affect 15–20% of the female population in the USA (Bachmann and Rosen, 2006). There is a limited number of studies on the use of BoNT for the treatment of vulvodynia (Brin and Vapnek, 1997; Shafik and El-Sibai, 2000; Gunter et al., 2004; Dykstra and Presthus, 2006; Bertolasi et al., 2009; Pelletier et al., 2011). Initial studies targeted overactive muscle sites in the vagina and pelvic floor with the intention of decreasing muscle spasm and, therefore, pain. Because recent research has suggested that BoNT may affect peripheral and central sensitization, researchers have targeted overactive muscle and painful tissue areas with the intention of relaxing muscle and inhibiting the release of neurotransmitters that can cause pain and inflammation (substance P and calcitonin gene-related peptide) (Dykstra and Presthus, 2006).
Injection techniques with BoNT for vulvodynia range from 10 U to 50 U onabotulinumtoxinA and 150 U to 400 U abobotulinumtoxinA. Dilutions of BoNT have ranged from 0.5 ml to 1.0 ml. Injection sites have included the anterior vaginal wall muscles, the puborectalis, pubococcygeus, perineal body, bulbocavernosus and bulbospongiosus muscle (Figs. 33.3 and 33.4). The number of injection sites in each muscle has varied from one to three. Needle size has been between 23- and 30-gauge. Patients had either no sedation or mild conscious sedation. Electromyography has been used in a few studies to locate the muscle being injected. Studies have been mainly single or multiple case series with single or multiple follow-up injections. One study was controlled (Shafik and El-Sibai, 2000), whereas all others were prospective cohort studies or case reports. All studies showed improvement in most patients regarding pain, muscle spasm, quality of life and sexual activity. Duration of effects lasted from 4 weeks to 2 years. A small number of patients were cured. No significant adverse effects were noted in any study. Therapy with BoNT is suggested for women with hyperactive pelvic floor at physical examination, or for those who respond poorly to oral drugs and/or surgical procedures. It should be noted that the actual level of evidence for use of BoNT in vulvodynia is low, and it is not currently recommended as a first-line treatment by current guidelines (Nunns et al., 2010).
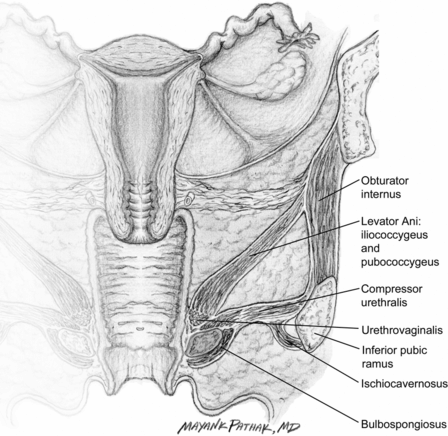
Fig. 33.3 Vaginal and pelvic floor muscles.
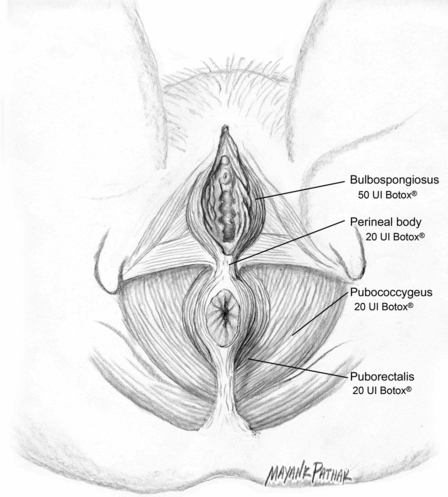
Fig. 33.4 Pelvic floor muscles.
Chronic pelvic pain
Non-malignant pain may be perceived in structures related to the pelvis of both males and females. Pain must have been continuous or recurrent for at least 6 months. If non-acute and central sensitization pain mechanisms are well documented, the pain may be regarded as chronic, irrespective of the time period. In all cases, there are often associated negative cognitive, behavioral, sexual and emotional consequences (Fall et al., 2010).
Approximately 15–20% of women aged 18–50 years have chronic pelvic pain of greater than 1 year in duration (an estimated number greater than for migraine, asthma and back pain) (Howard, 2003). Chronic pelvic pain may result from psychological disorders or neurological diseases, both central and peripheral. Sufficient evidence strongly suggests that several of the most common disorders in women, such as endometriosis, interstitial cystitis, irritable bowel syndrome and pelvic inflammatory disease, are causes of chronic pelvic pain. In men, chronic pelvic pain syndrome is an enigmatic medical condition that has been classified as category III chronic pelvic pain syndrome or non-bacterial chronic prostatitis, and, more recently, as prostate pain syndromes (Fall et al., 2010).
Patients with chronic pelvic pain may have generalized or localized pelvic pain, pain with intercourse, pain with ejaculation, pain exacerbation after sexual intercourse, pain exacerbated both premenstrually and menstrually and complaints of voiding symptoms of frequency, urgency and nicturia (Howard 2003).
There are a few studies on the use of BoNT for the treatment of chronic pelvic pain (Jarvis et al., 2004; Thomson et al., 2005; Abbot et al., 2006; Meredeth et al., 2006; Gottsch et al., 2011). These studies have included both male and female patients. Injection techniques with BoNT for chronic pelvic pain have ranged from 40 to 200 U onabotulinumtoxinA. Dilutions have ranged from 2 to 4 ml. Injection sites have included the puborectalis (20 to 50 U onabotulinumtoxinA), pubococcygeus (25 U onabotulinumtoxinA), the bulbospongiosus (25 U onabotulinumtoxinA), the perineal body (25 U onabotulinumtoxinA) and the external urethral sphincter muscles (Fig. 33.4). The number of injections into each muscle has ranged from three to five sites, and a 22-gauge needle is usually used. Patients have had either no sedation or only mild conscious sedation. Electromyography was used in two studies to improve muscle localization. Two studies were randomized and placebo controlled (Abbott et al., 2006; Gottsch et al., 2011), while the others were prospective cohort studies or case series. All studies showed improvement in most patients regarding pain, spasm, quality of life and sexual activity. Duration of effect was 3 to 18 months. No adverse effects were noted. There was a reduction in dyspareunia and non-menstrual pelvic pain, associated with a significant reduction in pelvic floor pressure, in the BoNT-treated group (Abbott et al., 2006) and a modest but significative reduction of the Global Response Assessment and of the pain subdomain of the Chronic Prostatitis Symptom Index in the BoNT group compared with placebo group (Gottsch et al., 2011).
Conclusions
The use of BoNT for the treatment of vulvodynia and chronic pelvic pain may be a viable option for patients. Adequately powered, controlled, double-blind studies are needed to further explore this treatment option.
Benign prostatic hyperplasia
Non-malignant enlargement of the prostate (BPH) is regarded as a major cause of bladder outlet obstruction. Because surgical denervation was known to produce profound atrophy in the rat prostate, onabotulinumtoxinA was used to show selective chemical denervation and subsequent atrophy in the rat prostate. The pathophysiology of BPH may involve a dynamic component that reflects the smooth muscle tone within the gland and a static component that is related to the mass effect of the enlarged prostate. Botulinum neurotoxin may have an effect on both components by relaxing smooth muscle and causing atrophy of the glandular tissue.
There have been a few studies on the use of BoNT in humans. Injections of 100–300 U onabotulinumtoxinA have been made at two to ten sites, with a 4–20 ml dilution factor. The injections were made into the transition zone at the lateral lobes and median lobes of the prostate or into both lateral lobes of the prostate (Fig. 33.5). Needle size was 21-, 22- or 23-gauge and injections were guided by rectal utrasound, transrectal ultrasound or cystoscope. The procedure was carried out without sedation or anesthesia, under light intravenous sedation or under general anesthesia. No meaningful difference was observed between using 100 or 300 U onabotulinumtoxinA (Crawford et al., 2011). Most studies were case series, while one was a double-blind, placebo-controlled randomized study (Maria et al., 2003).
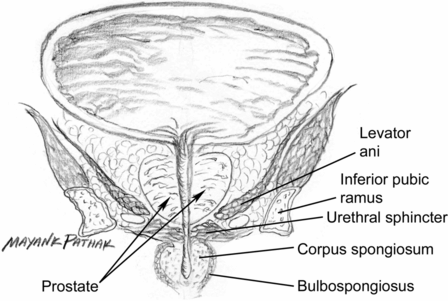
Fig. 33.5 Male genetalia and prostate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


