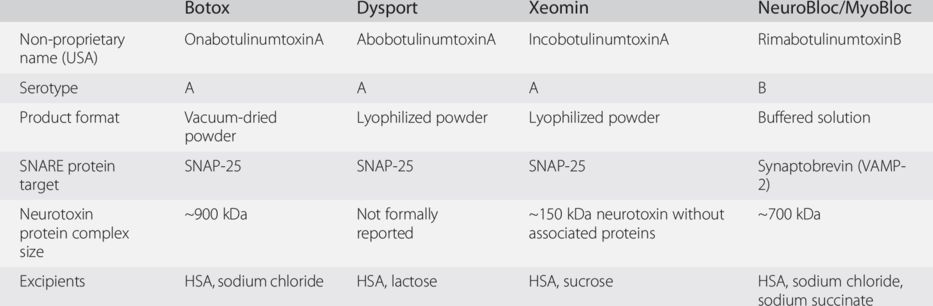
HSA, human serum albumin; SNAP-25, synaptosomal associated protein-25; VAMP-2, vesicle-associated membrane protein-2.
Onset and duration of action
Clinical effects of BoNTs develop gradually and are typically evident within a week of injection (Moore and Naumann, 2003). As noted previously, duration of action is approximately 3 to 4 months when administered into skeletal muscle and patients typically request reinjection around this time. Longer durations may be seen following injection into autonomic terminal regions, as in the case of overactive bladder and axillary hyperhidrosis.
Once the effects wear off, BoNTs may be reinjected and many patients with neurological conditions receive repeated BoNT treatment for years or decades. The response is typically maintained over many years (Lungu et al., 2011), although the injected muscles may need to be altered in response to changes in the pattern of muscle activity over time (Gelb et al., 1991).
Immunogenicity
As with most protein therapies, there is the potential for antibody formation with BoNT therapy. The extremely small amounts of BoNT protein needed to produce a biological effect likely help to minimize immunogenicity, but patients occasionally develop antibodies over time. Neutralizing antibodies can interfere with the clinical effects of BoNT (Brin et al., 2008) and patients may need to switch to a different serotype or different therapy altogether. Neutralizing antibody development may be influenced by the dose/amount of BoNT complex, frequency of injections, and choice of BoNT product, as well as by individual factors that have not been well characterized.
Before concluding that a patient’s non-response is caused by neutralizing antibodies, however, it may be prudent to consider other factors. Administrative errors, muscle selection, product storage or reconstitution errors, expectation effects or emotional/social factors related to the underlying physical illness may all influence clinical response (Moore and Naumann, 2003). Given these possibilities, some experts recommend that another injection cycle be tried at the same dose in patients who experience a reduced response, provided that side effects were not intolerable (Moore and Naumann, 2003). While there are serological tests available for antibodies, the best way to check is functionally with small injections into sites where the effect should be obvious. These sites include the unilateral brow, the extensor digitorum brevis muscle in the foot and the abductor digiti minimi in the hand.
Adverse events
In general, BoNT injections are well tolerated and show acceptable safety across a wide range of conditions and disorders (Naumann and Jankovic, 2004; Brin et al., 2009). The most frequent adverse events are local weaknesses in nearby muscles, and these tend to be mild or moderate in severity (Naumann and Jankovic, 2004). However, severe adverse events do occasionally occur with BoNTs and, therefore, it is always advisable to follow injection guidelines for each individual product and not to exceed the upper recommended doses for the product.
Because BoNTs have local actions within the injected regions, they do not typically interact with systemic medications. This is an advantage for all patients but particularly for those who are taking multiple medications to treat multisymptom conditions such as poststroke neurological damage.
All BoNT serotype A drugs have similar adverse effect profiles. The adverse effect profile of the BoNT serotype B drug (rimabotulinumtoxinB) is slightly different. The type B drug frequently produces autonomic adverse effects, including dryness of mouth (Dressler and Benecke, 2003); however, the frequency of motor adverse effects is similar after types A and B. Type B may have an advantage over type A in the treatment of autonomic disorders such as sialorrhea.
Future developments
The development of BoNT products is proceeding along several lines. First, manufacturers of current products are conducting clinical trials to expand the conditions for which they are indicated or approved in various regions of the world. The commonality among all of these conditions is that a focal reduction in cholinergic tone is beneficial. The exception to this rule may be the use of BoNTs for various conditions of pain, which is supported by preclinical evidence that BoNT serotype A inhibits the release of pain-related neurochemicals such as substance P and calcitonin gene-related peptide (Purkiss et al., 2000; Durham et al., 2004).
A second development is the increase in the number of different BoNT products. In addition to the products outlined in Table 3.1, which are all available in many countries worldwide, a number of other products are only available in certain regions, such as China. The lucrative facial esthetic market has also spurred the availability of counterfeit toxins, which are not approved in any country but are available via the Internet. It is important to note that these products have not undergone the necessary safety and biological activity testing required of approved products and, therefore, may be dangerous. Indeed, a 2006 report described four patients who received a highly concentrated, unlicensed BoNT preparation for cosmetic purposes (Chertow et al., 2006). These patients experienced serious side effects, illustrating the importance of using only licensed products at recommended doses.
A third path of BoNT development is the modification of the toxin molecule. For example, some scientists are attempting to engineer proteins that retain the endopeptidase activity of the toxin but possess an altered binding domain, such that the BoNT shows specificity for a different type of cell (Chen, 2012). The altered binding domain may also be coupled with a modified light chain designed to cleave non-neuronal SNARE proteins such as SNAP-23, which plays a role in the secretion of airway mucus in asthma (Chen, 2012). It is clear from these studies that much remains to be learned and gleaned from this interesting neurotoxin.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


