Urothelial carcinoma variants
Key morphologic features
Urothelial carcinoma with divergent differentiation
Urothelial carcinoma with squamous differentiation
Urothelial carcinoma with clear-cut squamous features including keratin production and/or presence of desmosomes
Urothelial carcinoma with glandular differentiation
Gland-forming urothelial carcinoma consisting of columnar, mucinous, signet ring, or intestinal-type cells
Urothelial carcinoma with deceptively benign appearance
Invasive urothelial carcinoma composed of cells with overall bland cytology; it exhibits the following architectures
Nested urothelial carcinoma
Small nests that resemble von Brunn’s nests
Large nested urothelial carcinoma
Large nests with pushing border, often connected to surface
Microcystic urothelial carcinoma
Small or large nests with lumina or cysts that resemble cystitis cystica
Urothelial carcinoma with small tubules
Tubular or acinar formation
Micropapillary urothelial carcinoma
Noninvasive surface filiform papillae that lack fibrovascular core and/or invasive small clusters of tightly packed cells in lacunar space with nuclei polarized exteriorly
Urothelial carcinomas with unusual cell morphology
Plasmacytoid urothelial carcinoma
Discohesive tumor cells with abundant cytoplasm and eccentric nuclei that resemble plasma cells
Urothelial carcinoma with rhabdoid features
Tumor cells with abundant cytoplasm and intracytoplasmic inclusion that indents and peripherally displaces the nucleus
Lipoid urothelial carcinoma
Tumor cells with large or multiple clear cytoplasmic vacuoles that indent the nuclei
Clear cell urothelial carcinoma
Carcinoma cells with clear cytoplasm that resemble clear cell renal cell carcinoma
Urothelial carcinoma with trophoblastic differentiation
Three types: (1) HCG production of morphologically urothelial carcinoma, (2) admixed trophoblasts in urothelial carcinoma, and (3) pure choriocarcinoma of bladder
Lymphoepithelioma-like carcinoma
Syncytium of high-grade undifferentiated cells in dense background of mainly lymphoplasmacytic inflammatory cells
Sarcomatoid urothelial carcinoma
Carcinoma with high-grade spindle cells or malignant heterologous elements (e.g., rhabdomyosarcoma, osteosarcoma, chondrosarcoma)
Urothelial carcinoma with unusual stromal reaction
Urothelial carcinoma with myxoid stroma and chordoid features
Abundant extracellular mucin and floating carcinoma cells exhibit varied patterns with no glandular differentiation
Undifferentiated urothelial carcinoma, osteoclast rich
Admixture of plump mononuclear cells and large multinucleated osteoclastic giant cells
Undifferentiated urothelial carcinoma
Undifferentiated carcinomas containing multinucleated anaplastic cells
Urothelial Carcinoma with Divergent Differentiation
Divergent differentiation in urothelial carcinoma is a common occurrence, with up to 27 % of urothelial carcinomas reported to show this feature, although the reported frequency varies depending on specimen type and study [1–4]. Most commonly, divergent differentiation is represented by squamous change within the neoplasm. The second most common form of divergent differentiation includes glandular change, identified as the presence of glands, small tubules, and occasionally signet ring cells or other glandular-like change. In addition to squamous and glandular differentiation, many other variants of urothelial carcinoma may be present admixed with conventional urothelial carcinoma.
Diagnostic criteria to report “urothelial carcinoma with squamous differentiation” entails identification of clear-cut squamous features within the tumor, including the presence of keratin or desmosomes (Fig. 15.1). These findings are present in a background of otherwise conventional urothelial carcinoma. Documentation of this finding is important, especially in instances where metastatic disease develops that may contain a squamous morphology . In urothelial carcinoma with squamous differentiation, the differential diagnosis includes squamous cell carcinoma of the bladder. The latter is distinguished by the presence of a pure squamous morphology, irrespective of the in situ component present on the surface [5]. In biopsy or transurethral resection specimens, this distinction is difficult since the entire lesion may not be represented in the material submitted [6]. In such instances of incomplete tumor sampling, where an extensive squamous cell carcinoma component is present, a comment should be included with the report that states, “urothelial carcinoma with extensive squamous differentiation cannot be excluded with certainty. Analysis of the entire lesion it is required to distinguish urothelial carcinoma with extensive squamous differentiation from pure squamous cell carcinoma of the bladder.” Although no diagnostic markers are uniformly useful in distinguishing these two entities, several studies have evaluated markers that can aid in identifying such lesions as being primary to the bladder . Specifically, S100P, GATA3, uroplakin III, cytokeratin 14, and desmoglein-3 have been shown to have utility in many cases in identifying a squamous predominant urothelial lesion as being primary to the bladder [7, 8].
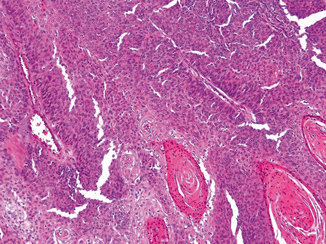

Fig. 15.1
Urothelial carcinoma with squamous differentiation including keratin pearl formation
The diagnosis of urothelial carcinoma with glandular differentiation is slightly less problematic. In general, the adenocarcinoma components can show glandular, mucinous, or signet ring cell differentiation in the background of conventional urothelial carcinoma (Fig. 15.2). The primary differential diagnosis in this instance includes either direct spread of a colonic carcinoma or metastatic spread from an adenocarcinoma at a different anatomic location. When approaching this differential, the finding of an in situ component and/or conventional urothelial carcinoma component is helpful in suggesting the lesion is primary to the bladder. In cases in which there is extensive adenocarcinomatous differentiation, a comment should be included stating “we cannot definitely exclude secondary spread from a non-bladder adenocarcinoma, such as colon cancer. Clinical and radiographic assessment of the patient is required.”
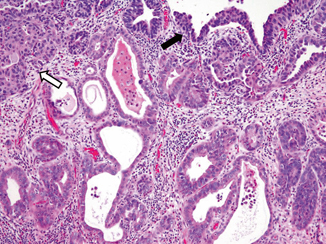

Fig. 15.2
Urothelial carcinoma with glandular differentiation. Note the presence of surface in situ ( dark arrow) and invasive ( open arrow) urothelial carcinoma. The malignant glands are lined by columnar cells
It remains somewhat unclear in the literature whether divergent differentiation suggests an overall worsened outcome for this patient population. In some studies, it has been suggested that correction for pathologic stage shows similar outcomes for conventional urothelial carcinoma and urothelial carcinoma with divergent differentiation whereas other studies suggest that patients with divergent differentiation have diminished outcomes [9–12]. Further patient-based studies that address this question are needed. A second clinical concern associated with urothelial carcinoma with divergent differentiation is response to therapy. Several studies have shown a reduced response to chemotherapy and radiation therapy; however, the relationship to morphology remains somewhat unclear [13, 14].
Deceptively Benign-Appearing Variants
Nested Urothelial Carcinoma
Archetypical for this group of innocuous-looking carcinomas is the nested variant , which at the surface closely resembles a benign von Brunn’s nest proliferation [15–24]. Diagnosis of nested variant can be very difficult in superficial bladder specimens. Nested variant is uncommon, encountered in 0.8–2.4 % of cancer cystectomies [16, 19]. The percentage of nested morphology for diagnosis has not been established, though some requires at least a 50 % component [16, 17]. Some tumors may be encountered as recurrence in patients with prior usual urothelial neoplasm. Most nested variant tumors present with a clinically recognizable bladder mass, of which about two thirds are locally aggressive [16] .
Nested urothelial carcinoma is characterized by haphazardly infiltrating nests of bland-appearing urothelial cells that often extends deeply into the muscularis propria (Fig. 15.3a, b). The tumor cells have modest eosinophilic cytoplasm and predominantly show minimal atypia and indistinct nucleoli, although scattered atypical cells are invariably present. Mitoses are typically rare. The nests are usually solid, well delineated, tightly packed, and may show confluence or fusion. The tumor cells may also be arranged into cordlike or trabecular patterns. Occasionally, small lumina may form within the nests producing cysts that may contain eosinophilic secretions and resemble cystitis cystica. Less commonly, focal tubules are also formed intermingled with the infiltrating nests. These microcysts and tubules are similar to those seen in microcytic urothelial carcinoma and urothelial carcinoma with small tubules discussed below. The intervening stroma between the infiltrating tumor nests often shows absent or only minimal tissue reaction. Lymphovascular invasion is often encountered, which can be a helpful hint in the diagnosis. At the deeper aspect of invasion, some neoplastic cells may show greater degree of cytologic atypia exhibiting larger, more irregular and hyperchromatic nuclei. Nested urothelial carcinoma is often encountered admixed with conventional urothelial carcinoma, the latter identified in more than half of tumors with at least 50 % nested morphology [17]. At the surface, no in situ or papillary carcinoma are often identified, where a von Brunn’s nest-like proliferation or ulceration is appreciated .
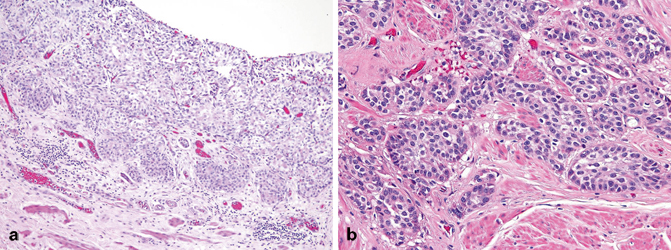

Fig. 15.3
Nested urothelial carcinoma (a) at the surface where it resembles von Brunn’s nests proliferation and (b) invading the muscularis propria
Poorer outcome in nested urothelial carcinoma is attributed to its tendency to high-stage presentation. Compared to conventional urothelial carcinoma, nested variant has a higher rate for locally aggressive disease and nodal involvement at 69–82 % and 19–57 %, respectively [16, 17]. However, when matched stage for stage, behavior of nested variant is not different from conventional urothelial carcinoma. A recent Mayo Clinic study showed a 10-year cancer-specific survival of 41 % for nested urothelial carcinoma versus 46 % for stage-matched conventional urothelial carcinoma [16] .
The main differential diagnoses for nested urothelial carcinoma are florid von Brunn’s nests and bladder paraganglioma. Morphologic similarities and differences between nested urothelial carcinoma and von Brunn’s nests are summarized in Table 15.2. Proliferation markers such as Ki67 and other markers such as p53, p27, and cytokeratin 20 are not helpful in discriminating these two tumors, except when Ki67 is markedly high (> 15 %), which may occur albeit only in a minority of nested urothelial carcinoma and not in von Brunn’s nests. Review of cystoscopy findings and close communication with urologists are crucial in the diagnosis of nested urothelial carcinoma, since most tumors present with a clinically appreciable mass concerning for cancer irreconcilable to a von Brunn’s nests consideration.
Table 15.2
Histological features of nested urothelial carcinoma and florid von Brunn’s nests
Nested urothelial carcinoma | Florid von Brunn’s nests | |
|---|---|---|
Distribution | Haphazard | Orderly |
Nests | Some tightly packed, confluent, or fused | Well spaced with regular boundary |
Associated microcysts | May be present | May be present (i.e., cystitis cystica) |
Associated well-differentiated glands | Absent | May be present (i.e., cystitis glandularis) |
Surface in situ or papillary carcinoma | Uncommon | Absent |
Cytologic atypia | Random atypia near surface and more conspicuous at deeper aspect | Absent |
Muscularis propria invasion | Common | Absent |
The “zellballen” pattern of bladder paraganglioma mimics the infiltrating nests of nested urothelial carcinoma. Knowledge of clinical information is important, since paraganglioma tends to occur in a wider age range including patients younger than the ones typically affected by urothelial carcinoma, and presents with micturition-associated hypertension, headache, or dizziness. Bladder paragangliomas are often centered deep in the muscularis propria and may not involve the surface (bottom-heavy). Bladder paraganglioma expresses the neuroendocrine markers synaptophysin, chromogranin and CD56, and the intratumoral sustentacular cells can be highlighted by S100 .
Large Nested Urothelial Carcinoma
Large nested urothelial carcinoma is characterized by medium-to-large invasive nests with pushing border akin to that of a verrucous carcinoma (Fig. 15.4). A series of 23 cases was reported from John Hopkins Hospital in patients 39–89 years old who were mostly men [25]. Similar to nested urothelial carcinoma, this variant is mainly composed of urothelial cells that lack significant cytologic atypia. Likewise, the tumor nests may also form focal cysts and tubules. Unlike nested urothelial carcinoma, the invasive nests are larger, usually connected to the surface, and separated by intervening stroma; lymphovascular invasion is not common. In addition, a surface papillary urothelial neoplasm component is more often present, seen in 83 % of tumors. The significance of this morphology in terms of tumor biology and behavior is still unclear. Most reported cases were diagnosed with (at least) deep muscle-invasive disease, suggesting similar behavior to nested urothelial carcinoma . The differential diagnosis includes von Brunn’s nests and inverted papillary urothelial carcinoma. Unlike von Brunn’s nests and inverted papillary urothelial carcinoma , large nested urothelial carcinoma has more variable and irregular nests, have intervening stromal reaction, and may extend deep into the muscularis propria. Presence of a surface papillary neoplasm component is helpful to distinguish it from von Brunn’s nests.
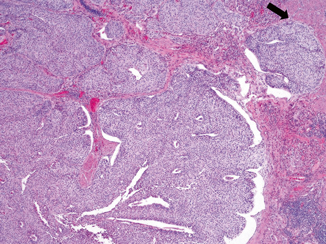

Fig. 15.4
Large nested urothelial carcinoma exhibiting broad front infiltration with involvement of muscularis propria ( arrow)
Microcystic Urothelial Carcinoma
This variant is composed of invasive small and large cysts of bland-appearing cells that closely resemble cystitis cystica (Fig. 15.5) [26–30]. As mentioned above, similar cysts are sometimes seen focally in nested and large nested urothelial carcinomas. There is no established cut-off for the proportion of cysts necessary for the diagnosis. Some authors have proposed at least 25 % cysts formations [31]. The cysts can be small or large (1–2 mm) comprised of transitional cells and occasional flattened cells. Focal mucinous cell change has also been described. Columnar cells are typically not present. The lumens of the microcysts are often filled with eosinophilic secretions or necrotic cellular debris. Behavior of this variant is not known, and probably is similar to nested urothelial carcinoma. Microcystic urothelial carcinoma can be distinguished from cystitis cystica by its more haphazard, variable, and irregular nests, and extension into the muscularis propria.
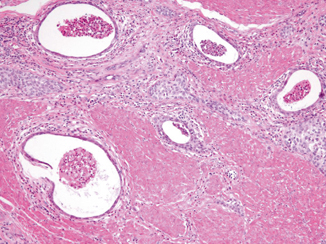

Fig. 15.5
Microcystic urothelial carcinoma invading muscularis propria
Urothelial Carcinoma with Small Tubules
Similar to cysts, small tubular or acinar formations may be present focally in nested and large nested urothelial carcinomas (Fig. 15.6). When the invasive tubules predominate, diagnosis of urothelial carcinoma with small tubules is rendered. This variant is very rare with less than a dozen cases reported [26, 32]. The tubules are composed mainly of bland-appearing cuboidal cells with random pleomorphism, similar to nested urothelial carcinoma . Tall columnar cells are generally not appreciated in these tubules. The tubules infiltrate haphazardly and often invade into the muscularis propria. The clinical significance of this variant is unknown, and probably is similar to nested urothelial carcinoma.
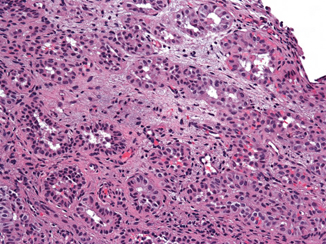

Fig. 15.6
Nested urothelial carcinoma with focal tubular formation
The main differential diagnoses of urothelial carcinoma with small tubules include tubules of nephrogenic adenoma, cystitis glandularis, Gleason grade 3 prostatic adenocarcinoma, and primary bladder adenocarcinoma. Careful search for non-tubular urothelial carcinoma component is helpful to distinguish from nephrogenic adenoma and prostate adenocarcinoma. Nephrogenic adenoma is typically superficial and usually has a surface papillary component lined by a single layer of cuboidal, flat, or hobnail cells. The diagnosis of nephrogenic adenoma can be confirmed by Pax2, Pax8, and S100P positivity, and negativity for GATA3 or p63. Prostate adenocarcinoma can be confirmed by PSA, PAP, NKX3.1, or PSMA positivity. Both cystitis glandularis and primary adenocarcinoma are lined by taller columnar cells and may show Goblet cell or mucinous change. Adenocarcinoma exhibits greater degree of cytologic atypia than urothelial carcinoma with small tubules.
Micropapillary Urothelial Carcinoma
Micropapillary urothelial carcinoma has received significant attention in recent years due to both distinctive pathology features as well as aggressive biologic behavior. Whereas, micropapillary urothelial carcinoma has anecdotally been considered to present at higher pathological stage, a recent study from MD Anderson has confirmed the importance of this clinical diagnosis, reported on the aggressive nature of this variant, and suggested early therapy (radical cystectomy) to be critical to improve patient outcomes [33].
Micropapillary urothelial carcinoma shares morphologic features with micropapillary carcinomas from other sites [34–36]. Specifically, the neoplastic cells form small tight clusters lacking a central fibrovascular core, with the nuclei of the tumor cells polarized to the exterior surface of the clusters (Fig. 15.7a, b). Surrounding the tumor cells is a prominent retraction artifact that gives the appearance of small epithelial nests floating in empty spaces. An in situ variant of micropapillary urothelial carcinoma has been reported, which has been described as thin, filiform exophytic processes on the bladder surface that may be highly branched and glomeruloid on cross section. Despite a relatively clear-cut definition, one recent study has shown that there is significant interobserver variability in the diagnosis of this entity [37]. Although not standard practice at many locations, some studies have shown that the extent of micropapillary urothelial carcinoma present in the background of an otherwise conventional urothelial carcinoma may have prognostic significance; however, this finding varies across studies [38, 39]. Based on these findings, the current convention holds that micropapillary differentiation should be reported regardless of the percentage of this variant present in the tumor [38].
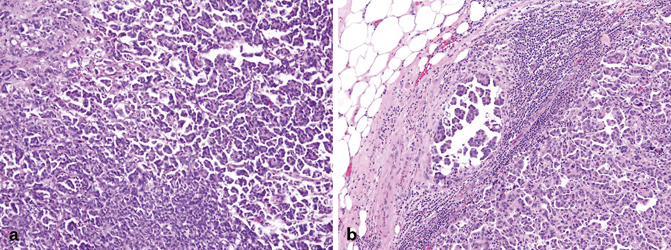

Fig. 15.7
Micropapillary urothelial carcinoma (a) shows small tight clusters of urothelial carcinoma cells in retraction spaces. b Lymph node metastasis retains the micropapillary architecture
It is important in biopsy and transurethral resection specimens to carefully assess for depth of tumor invasion, as published reports have demonstrated that many of these carcinomas are muscle-invasive (pT2) at the time of diagnosis [34, 38]. Thus, it is critical to report when muscularis propria (detrusor muscle) is absent in the specimen; in such cases, a comment that states “micropapillary urothelial carcinoma may commonly invade the muscularis propria; additional sampling to evaluate muscle invasion is recommended” should be included. In addition, many of these tumors show angiolymphatic invasion that should also be documented in the final pathology report [34] .
The major differential diagnoses for this variant includes urothelial carcinoma with extensive retraction artifact and metastatic carcinoma with micropapillary morphology , such as papillary serous carcinoma of the ovary. In the latter instance, positive immunohistochemistry for uroplakin and CK20 may aid in the final diagnosis, especially when correlated with patient history and imaging [40]. Additional ancillary tests, including MUC1, HER2, and CA125, have been studied in micropapillary urothelial carcinoma; however, these markers may not reliably distinguish all micropapillary urothelial carcinomas from conventional urothelial carcinoma with extensive retraction artifact [41, 42].
A diagnosis of micropapillary urothelial carcinoma generally denotes that the tumor will behave aggressively. This is supported by the study from MD Anderson that showed that not only does this tumor type respond less well to Bacillus Calmette-Guerin (BCG) and chemotherapy, but that 67 % of patients had progression with 22 % of patients developing metastatic disease [43]. Based on this study, many sites now advocate for early cystectomy in patients with micropapillary urothelial carcinoma. However, according to a recent study from Memorial Sloan-Kettering Cancer Center a subset of patients with cT1 micropapillary urothelial carcinoma managed conservatively were not found to have significantly worse outcomes compared to patients undergoing early radical cystectomy [44]. It is clear that, based on the inherent challenges in the diagnosis of this entity, further collaborative efforts and research into identifying objective markers for diagnosis are needed.
Plasmacytoid Urothelial Carcinoma
This variant has an unusual composition of infiltrative discohesive cells with eccentrically placed nucleus that resemble plasma cells and poorly differentiated carcinomas [45–55]. The complexity in diagnosis is confounded by their immunopositivity to the usual plasma cell marker CD138. Reported prevalence of this tumor is about 1 % among high-grade urothelial carcinomas and 2.7–3.0 % of muscle-invasive urothelial carcinomas [53–55]. The tumor may present purely with plasmacytoid morphology , although more often, it is encountered admixed with high-grade conventional urothelial carcinoma. The amount of plasmacytoid morphology varies in published series, with most reports using a 30 or 50 % cut-off [45, 47, 50–52] .
Although a discrete bladder mass can often be cystoscopically detected, this tumor can also diffusely infiltrate and thicken the bladder wall in a linitis plastica-like manner. The tumor infiltrates the bladder as cords, small nests, or sheet-like growths (Fig. 15.8). Infiltrative cells may also be in a single cell pattern reminiscent of invasive lobular carcinoma of the breast. The tumor cells contain modest to abundant amphophilic to eosinophilic cytoplasm, with low- to intermediate-grade nuclei distinctively displaced to one side. Overall, there is some degree of monotony of the tumor cell infiltrates. Occasionally, intracytoplasmic mucin may be focally present and may loosely resemble signet ring cells [50]. The background stroma often appears edematous or myxoid.
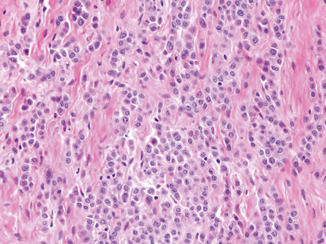

Fig. 15.8
Plasmacytoid urothelial carcinoma
Interestingly, one study showed that FGFR3 and PIK3CA mutations, common in invasive urothelial carcinoma that arises from papillary neoplasm, are not detected in plasmacytoid variant [46]. Unlike conventional urothelial carcinoma, E-cadherin expression is often completely lost in plasmacytoid variant including its concomitant conventional type [46].
Most plasmacytoid urothelial carcinoma presents at a higher stage. A study from MD Anderson reported 85 % of patients with at least muscularis propria invasion and 48 % with metastasis or locally unresectable tumors [47]. The high-stage presentation translates to a poor outcome with a reported median survival of only 17.7 months. Local spread and recurrence is rather unique among urothelial carcinomas where it occurs most commonly in serosal surface including the peritoneum, where it may present as carcinomatosis [47, 48]. Survival of patients with metastatic disease is poor despite of chemotherapy .
The main differential diagnosis for plasmacytoid urothelial carcinoma includes chronic cystitis (or inflammation), plasma cell neoplasm, metastatic poorly differentiated carcinomas, particularly gastric signet ring cell carcinoma and lobular carcinoma of the breast, and primary bladder signet ring cell adenocarcinoma. Diagnosis can be challenging at metastatic sites. Although the plasmacytoid variant is frequently CD138 positive, it does not exhibit kappa or lambda restriction. Keratin AE1/AE3, CK7, or CK20 expression helps distinguish plasmacytoid urothelial carcinoma from hematopoietic cells. It is highly unusual for gastric and breast carcinomas to present as an isolated finding in the urinary tract; when encountered, they are usually part of a widely disseminated disease. The presence of admixed conventional urothelial carcinoma, including the surface in situ component, is helpful in establishing the diagnosis of plasmacytoid urothelial carcinoma. Plasmacytoid variant expresses the urothelial-associated marker GATA3, which may help in the differential diagnosis [49]. Primary signet ring cell adenocarcinoma exhibits predominance of tumor cells with intracytoplasmic mucin, which is only focally present in plasmacytoid urothelial carcinoma.
Urothelial Carcinoma with Rhabdoid Features
This exceedingly rare morphology typically occurs in association with poorly differentiated urothelial carcinomas. Rare examples of “pure” malignant rhabdoid tumors of the bladder have been described in pediatric patients [56–59]. In adults , the only series reported four cases in the bladder of men ages 53–86 years [60]. Rhabdoid morphology comprised at least 60 % of the tumors that also had an in situ or papillary urothelial carcinoma component [60]. The rhabdoid cells are discohesive, infiltrate as single cells, small nests, or diffuse sheets (Fig. 15.9). The tumor cells are plump oval to round with abundant cytoplasm containing intracytoplasmic inclusion that displaces the nucleus. The nuclei are large with vesicular chromatin and prominent nucleoli. High-grade undifferentiated morphology such as small cell and sarcomatoid carcinoma may coexist. Keratin expression (dot-like or diffuse) and negativity for myogenic markers such as desmin, myoD1, or myogenin help establish the epithelial lineage. Ultrastructurally, the cytoplasmic inclusion was shown to be whorls of intermediate filament. In non-vesical carcinomas, the presence of rhabdoid morphology has been associated with poor outcome. The few reported cases of urothelial carcinoma with rhabdoid features suggest an aggressive behavior.
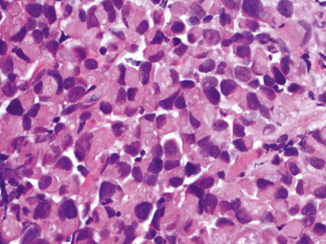

Fig. 15.9
Urothelial carcinoma with rhabdoid features
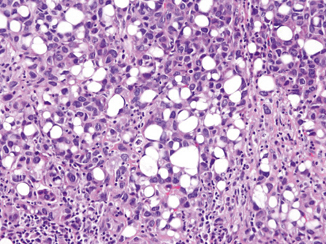
Lipoid (Lipid-Rich) Urothelial Carcinoma
This variant is characterized by presence of large cells containing large or multiple clear vacuoles that indent the nucleus to resemble lipoblasts or signet ring cells (Fig. 15.10). There are about 35 examples of this variant reported in the literature [61–65]. Almost always, the lipoid carcinoma is admixed with conventional or other variants of urothelial carcinoma. The lipoid cells comprise 10–50 % of the tumor. The overall tumor growth architecture on low-power magnification is similar to conventional urothelial carcinoma. The lipoid cells nuclei show moderate and occasional pleomorphism. The cytoplasmic vacuoles are optically clear and have been shown to have no mucin content. The immunoprofile is similar to that of conventional urothelial carcinoma. Most patients present with higher stage disease, which contributes to a poorer outcome. In a multi-institutional study of 27 cases, 89 % of lipoid urothelial carcinomas were at least deep muscle invasive, 45 % had lymph node metastasis, and 60 % died of disease within 58 months [62].