Biomarker
Functional role
Clinical application
Category
AMACR
β-oxidation of branched chain fatty acids
Diagnostic
Tissue
TMPRSS2:ERG gene fusions
Oncogenic transcription factor
Diagnostic, prognostic, predictive
Tissue or urine
GSTP1
Caretaker gene
Diagnostic
Tissue
PCA3
Prostate cancer-specific marker, produces PCA3 RNA with no resultant protein
Diagnostic, prognostic
Urine
PCA3 + ERG fusions
See PCA3 and ERG
Diagnostic, prognostic
Urine
PTEN
Tumor-suppressor gene
Prognostic, predictive
Tissue
EZH2
Transcriptional memory
Prognostic
Tissue
SPINK1
Functional role in ETS rearrangement-negative prostate cancer
Prognostic, diagnostic
Tissue, urine
Molecular Markers of Diagnosis
Tissue-Based Molecular Markers of Diagnosis
α-Methylacyl-CoA Racemase
Biology
AMACR was discovered as a leading candidate gene by differential display and complementary deoxyribonucleic acid (DNA) subtraction microarray analysis [1, 2]. It is consistently overexpressed in prostate cancer compared to benign prostatic tissue [1, 2]. It encodes a cytoplasmic protein involved in the β-oxidation of branched chain fatty acids. AMACR is not prostate cancer specific; it is also expressed by other cancers most notably, colorectal carcinomas and renal cell carcinomas , papillary type [3] .
Clinical Applications
Both monoclonal and polyclonal antibodies to AMACR have been developed. P504S, commercially available monoclonal antibody to AMACR, is most widely used, clinically. In our experience both monoclonal and polyclonal antibodies demonstrate similar levels of sensitivity and specificity for prostate cancer [4]. AMACR expression is cytoplasmic with granular staining pattern. The staining shows apical predominance (Fig. 9.1) and frequent heterogeneity (Fig. 9.2). Proportion of benign glands may also express AMACR; therefore interpretation of AMACR expression must be evaluated relative to the background staining of benign glands in the same biopsy. If benign glands demonstrate similar intensity of staining compared to atypical glands, then staining should be interpreted as negative.
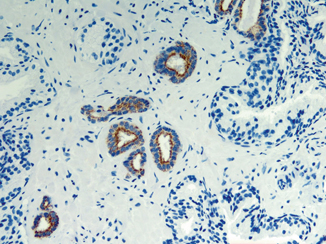
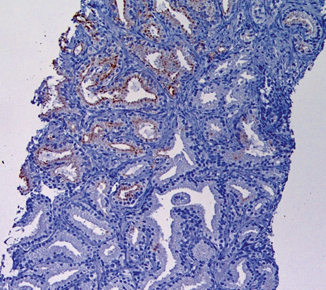

Fig. 9.1
An example of limited prostate carcinoma immunostained with P504S monoclonal antibody to AMACR (× 200). Expression of AMACR appears as granular staining predominantly in apical portion of cancer glands. Adjacent benign glands are negative for AMACR

Fig. 9.2
Heterogeneous AMACR staining in cancer glands. The staining is strong in some cancer glands and weak or negative in others. This staining pattern is typical in prostate cancer and accounts for an 80 % AMACR positive rate in prostate cancer detected on needle biopsy
AMACR in the Diagnosis of Limited Prostate Cancer in Prostate Needle Biopsy
AMACR was the first prostate cancer tissue biomarker identified. Currently, AMACR is more commonly applied to complement basal cell markers in antibody cocktail formats. The cocktails are now routinely utilized to resolve the diagnosis of “atypical glands” or to confirm the diagnosis of small volume cancer in needle biopsies (Fig. 9.1) [5]. Average sensitivity for the detection of limited prostate carcinoma in needle biopsies is in the range of 70–80 % with lower sensitivity reported in certain morphologic variants including foamy, pseudohyperplastic, and atrophic variants of usual acinar prostate adenocarcinoma [6, 7]. AMACR is expressed in ~ 90 % of irradiated prostate carcinomas; its expression is reduced in hormone-deprived cancers [8].
Positive AMACR staining supports a diagnosis of cancer in morphologically suspicious atypical glands. A diagnosis of cancer should not be reversed if AMACR and basal cell markers are negative and the morphology of the atypical glands is suspicious for carcinoma as a small proportion of prostate cancer lacks AMACR expression.
Utility of AMACR in Resolving an “Atypical Glands Suspicious for Prostate Cancer” Diagnosis
One of the challenges encountered during biopsy evaluation is the diagnosis of atypical glands suspicious for cancer (ATYP), which typically require immunohistochemistry (IHC) for further diagnostic work up. Two studies have demonstrated that in a subset of ATYP cases, positive AMACR may convert an ATYP to cancer diagnosis where morphology is suspicious for but not diagnostic of cancer and basal cell markers are negative [6, 9]. Caution should be exercised, however, before making a cancer diagnosis based on AMACR positivity alone as AMACR has significant limitations with prostate cancer specificity. The diagnosis of high-grade prostatic intraepithelial neoplasia (HGPIN), partial atrophy, adenosis (atypical adenomatous hyperplasia) and nephrogenic adenoma must be ruled out on morphological grounds before making a diagnosis of limited prostate cancer based on AMACR positivity.
Pitfalls
AMACR is not entirely specific for prostate cancer detection. The majority of HGPIN, nephrogenic adenomas, a subset of partial atrophy lesions, adenosis, and even benign glands may demonstrate AMACR expression [6, 10–12]. A summary of biology, clinical applications, and pitfalls of AMACR biomarker is summarized in Table 9.2.
Table 9.2
α-Methylacyl-CoA racemase (AMACR, P504S)
Types of antibodies |
Monoclonal (P504S) and polyclonal, both with comparable sensitivity and specificity |
Staining pattern in prostate cancer |
Granular cytoplasmic staining in apical distribution pattern |
Diagnostic utility |
1. Positive AMACR staining in atypical glands morphologically suspicious for cancer supports a cancer diagnosis |
2. Positive AMACR may convert an ATYP to cancer diagnosis where morphology is suspicious but not diagnostic of cancer and basal cell markers are negative |
Pitfalls |
Staining intensity often heterogeneous in cancer glands |
~ 20 % of prostate carcinomas diagnosed on needle biopsy lack AMACR expression |
Lower positive rate in several histologic variants (foamy gland, atrophic, and pseudohyperplastic) of prostate carcinoma |
Positive in > 90 % HGPIN, 20 % adenosis, majority of nephrogenic adenomas; frequently positive in partial atrophy and morphologically benign glands |
Expressed in nonprostatic tumors (urothelial carcinoma, colon cancer, renal cell carcinoma, clear cell adenocarcinoma) |
TMPRSS2:ERG Gene Fusions
Biology of ETS Gene Fusions in Prostate Cancer
Recurrent chromosomal rearrangements in prostate carcinoma were discovered through an unconventional bioinformatics approach termed as the “Cancer Outlier Profile Analysis” (COPA) algorithm used to analyze DNA microarray studies. Using the results of COPA analysis of many prostate cancer profiling studies, in 2005 Tomlins et al. discovered recurrent chromosomal rearrangements in prostate cancer demonstrating fusion of the 5′ untranslated region of the androgen-regulated gene TMPRSS2 with ERG or ETV1, two members of the E26 transformation-specific ( ETS) transcription factor family genes [13]. Many subsequent studies have validated ETS gene fusions in the majority (~ 50 %) of PSA-screened prostate cancer surgical cohorts [14–17]. Fusions between TMPRSS2 and ERG represent the most common molecular subtype accounting for ~ 90 % of all ETS gene fusions [13, 14, 16, 17]. In addition to the most common TMPRSS2:ERG rearrangements, several other novel 5′ promoter or other upstream sequences of androgen-inducible genes ( HERV_K22q11.23, SLC45A3, C15orf21, HNRPA2B1, KLK2, CANT1) and 3′ ETS transcription factors genes ( ETV4, ETV5, and ELK4) have also been identified, which comprise about 5–10 % of all gene fusions in prostate cancers [17–19]. Rearrangements of ERG at the chromosomal level are highly specific to prostate cancer and are an early molecular event seen in ~ 18 % of HGPIN lesions immediately adjacent to cancer demonstrating identical gene fusions [15, 20]. HGPIN lesions expressing TMPRSS2:ETS rearrangements are invariably associated with invasive cancer, suggesting that they are a subset of true neoplastic precursors for TMPRSS2:ETS-positive cancers [15, 17, 20]. Clinically localized prostate cancer is typically a multifocal disease, with heterogeneous rearrangement for TMPRSS2:ETS fusions between different tumor foci [21]. In this schema of multifocal disease, a primary focus rearranged for TMPRSS2:ETS may progress and become capable of dissemination and give rise to metastatic disease. All metastatic disease foci retain similar TMPRSS2:ETS rearrangement like the primary focus, indicating that ETS rearrangement occurs before progression to metastatic disease and that the metastatic disease arises through the clonal expansion of a single focus of primary cancer capable of dissemination [22]. In summary, ETS gene fusions have been implicated to play a critical role in prostate carcinogenesis. ERG gene fusions have yet not been demonstrated in benign prostate tissue, isolated HGPIN, or benign cancer mimics [23–25]. Taken together, ERG gene fusions are the best prostate cancer-specific biomarker yet identified and define a specific molecular subtype of prostate cancer with important implications in diagnosis and management .
Anti-ERG Antibody as a Surrogate for ERG Gene Fusions in Prostate Cancer
TMPRSS2:ERG gene fusions result in the overexpression of chimeric fusion transcripts that encode a truncated ERG protein product. Park et al. characterized a rabbit anti-ERG monoclonal antibody [26]. A positive immunostain with this antibody highly correlated with the ERG gene rearrangement status determined by fluorescent in situ hybridization (FISH), with 96 % sensitivity and specificity for determining ERG rearrangement in prostate cancer. Several subsequent studies have validated this observation and demonstrated that ERG immunohistochemical expression has a high accuracy for defining the TMPRSS2:ERG fusion status in prostate cancer [27–31]. Table 9.3 summarizes the published studies highlighting the type of ERG antibody utilized, frequency of expression in prostate cancer, and its sensitivity and specificity for the detection of ERG gene fusions in prostate carcinoma. In summary, ERG oncoprotein detection in prostate cancer is highly concordant with ERG gene fusion status and can be reliably utilized as a surrogate of ERG gene fusions in prostate cancer diagnosis and management.
Table 9.3
Published studies demonstrating the frequency of ERG positivity and sensitivity and specificity of the detection of underlying ERG gene fusions in prostate carcinoma for two classes of ERG antibody
Type of antibody | Source of ERG antibody | Frequency of ERG positivity (%) | Detection of underlying ERG fusions | Study | |
|---|---|---|---|---|---|
Sensitivity (%) | Specificity (%) | ||||
C-terminus (clone EPR3864) | Epitomics | 44 | 96 | 97 | Park et al. |
N-terminus (CPDR ERG-MAb) | Noncommerciala | Concordance rate of 82.8 %b | Furusato et al. | ||
C-terminus (clone EPR3864) | Epitomics | 45 | 86 | 89 | Chaux et al. |
C-terminus (clone EPR3864) | Epitomics | 61 | 100 | 85 | Leenders et al. |
C-terminus (clone EPR3864) | Epitomics | 33 | 96 | 99 | Falzarano et al. |
C-terminus (clone EPR3864) | Epitomics | 45 | 96 | 99 | Braun M et al. |
N-terminus (clone 9FY) | Biocare | 45 | 98 | 98 | Braun M et al. |
Clinical Applications of ERG IHC
Two monoclonal antibodies to C-terminus (clone EPR3864) and N-terminus (9FY) have been developed and are now commercially available. A recent study demonstrated similar levels of sensitivity and specificity for detecting the ERG gene fusions for the two monoclonal antibodies [30]. Overall, C-terminus antibody clone EPR3864 has been the most widely utilized ERG antibody in published studies (Table 9.3). The vascular endothelial cells present ubiquitously in prostate biopsy are utilized as internal positive control (Fig. 9.3). ERG expression in cancer cell nuclei is typically diffuse and strong (Fig. 9.3) [23–25]. Heterogeneous staining within the same cancer focus is relatively uncommon but heterogeneity of staining between different tumor foci of multifocal cancer is frequently reported [21, 25].
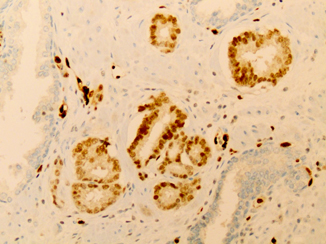

Fig. 9.3
An example of limited prostate carcinoma immunostained with ERG antibody (× 200). The vascular endothelial cells ( asterisk) that are present ubiquitously in prostate biopsy demonstrate strong nuclear staining and are utilized as an internal control. Cancerous glands demonstrate uniform strong nuclear ERG ( arrow) reactivity. Adjacent benign glands are negative for ERG
ERG in the Diagnosis of Limited PCa in Prostate Needle Biopsy
Basal cell markers including high-molecular weight cytokeratin and p63, and prostate cancer marker AMACR (P504S), individually or as a part of PIN-4 cocktail, are currently the most commonly utilized IHC markers in clinical practice [32, 33]. AMACR is preferentially overexpressed in approximately 80 % of prostate cancer detected in prostate biopsies [5, 6, 32, 33]. However, its expression is also found in most cases of HGPIN, in a significant proportion of adenosis, nephrogenic adenoma and partial atrophy, and occasionally even in morphologically benign prostatic glands [6, 10–12]. Therefore, a tumor marker that demonstrates better specificity for prostate cancer and is not expressed in noncancerous lesions may complement basal cell markers and AMACR and will greatly facilitate the identification of limited cancer in prostate biopsies.
Several studies have analyzed the utility of ERG immunostain in the work up of limited prostate cancer and have consistently found high specificity of ERG for prostate cancer detection. The reported frequency of ERG expression in limited cancer ranges from 40 to 60 % [23–25, 27, 29, 34, 35]. ERG expression has been observed in a small proportion of HGPIN or benign glands, invariably associated with adjacent prostate cancer [23–25, 34]. Benign lesions distant from cancerous glands, including simple and partial atrophy, are typically negative for ERG. Overall, ERG has much higher specificity for prostate cancer than AMACR; hence, ERG staining in an atypical focus (where HGPIN or atypical glands adjacent to HGPIN can be excluded) supports a diagnosis of cancer, irrespective of AMACR staining. A representative example of limited prostate cancer stained with ERG antibody is represented in Fig. 9.3.
Utility of ERG in Resolving an “Atypical Glands Suspicious for Prostate Cancer” Diagnosis
Only a few studies have examined the significance of ERG in the setting of ATYP [23, 24]. We studied 84 ATYP cases using multiplex ERG/AMACR/high molecular weight cytokeratin/p63 IHC to determine clinical utility of ERG in resolving an ATYP diagnosis [23]. A final diagnosis of benign , ATYP and cancer was rendered following review of morphology and all markers in 3, 30, and 51 cases, respectively. Of 51 cancer diagnoses, 45 and 94 % were positive for ERG and AMACR, respectively. Of 30 atypical diagnoses, 10 and 67 % were positive for ERG and AMACR, respectively. Of three benign diagnoses, none and 83 % were positive for ERG and AMACR, respectively. All three ERG-positive atypical cases were classified as “HGPIN with adjacent ATYP.” ERG was expressed in adjacent noncancer glands of 20 % of prostate cancers, while AMACR was expressed in noncancer glands in all diagnostic categories in 40 % of cases. In ERG-positive ATYP focus, the expression was predominantly uniform within the focus with minimal staining heterogeneity. Overall, ERG has a low sensitivity but high specificity for prostate cancer detection. Therefore, ERG positivity in small atypical glands where the diagnosis of HGPIN is excluded can be utilized to establish a definitive cancer diagnosis in the majority of ATYP cases.
Utility of ERG in Resolving an ATYP Diagnosis Beyond that Provided by Traditional AMACR and Basal Cell Markers
An important clinical question remains: is a positive ERG staining used merely to confirm a malignant diagnosis that could otherwise be established based on routine hematoxylin and eosin (H&E) histology and traditionally utilized PIN-4 cocktail antibodies composed of AMACR and basal cell markers? Alternatively, could a positive ERG staining be used to convert an atypical diagnosis to cancer in cases that otherwise would not be diagnostic of prostate cancer based on histology and traditionally utilized AMACR and basal cell markers? In our experience addressed in the earlier section of utility of ERG in resolving an “ATYP” diagnosis, traditionally utilized AMACR and basal markers were adequate to resolve ATYP diagnosis in the vast majority of cases [23]. However, owing to the high specificity of ERG for prostate cancer, ERG positivity in small atypical glands where the diagnosis of HGPIN was excluded helped establish a definitive cancer diagnosis in small proportion of additional ATYP cases where either morphology and/or traditional markers were not deemed adequate enough to offer a definitive cancer diagnosis. In this series, 12/48 (28 %) atypical diagnoses based on morphology, AMACR, and basal cell markers were changed to cancer after incorporating a positive ERG staining. These cases were morphologically suspicious for cancer and in all cases AMACR was expressed but a definitive diagnosis of cancer could not be rendered due to either quantitatively or qualitatively less than optimal morphology or inconclusive basal cell IHC. A positive AMACR staining was not sufficient in these cases to make a definitive cancer diagnosis as AMACR is also known to be expressed in a significant proportion of benign prostate cancer mimics. In this study, 67 % of lesions which were classified as atypical and 40 % of noncancer glands in all diagnostic categories demonstrated AMACR expression, indicating that AMACR expression is not cancer specific and by itself is not sufficient to convert an atypical diagnosis to cancer. On the contrary, ERG expression in small proportion of benign or HGPIN glands has been predominantly reported in the setting of adjacent cancer, therefore ERG positivity in small atypical glands, where the diagnosis of PINATYP or HGPIN is excluded, is virtually diagnostic of cancer [23, 24]. An example of “ATYP” (diagnosis based on morphology, AMACR, and basal cell markers) due to qualitatively less than optimal morphological features converted to cancer after incorporating positive ERG staining is represented in Fig. 9.4a–c.
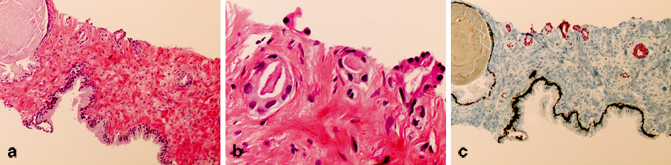

Fig. 9.4
An example of “atypical glands suspicious for cancer” (diagnosis based on morphology, AMACR, and basal cell markers) due to qualitatively less than optimal morphological features converted to cancer after incorporating positive ERG staining. The atypical glands are partially atrophic, lack obvious infiltrative architecture (a, × 200) and prominent atypia at higher magnification (b, × 400) required to render a definitive cancer diagnosis despite lack of basal cell staining and AMACR expression. The presence of strong diffuse nuclear ERG staining in atypical glands as demonstrated by PIN4-ERG multiplex stains supports the cancer diagnosis.
ERG Expressing HGPIN and ATYP Lesions as a Predictive Biomarker of Prostate Cancer Risk Stratification in Subsequent Prostate Biopsy
Previous FISH-based evaluations of the genomic rearrangements associated with prostate cancer development have consistently revealed that about 20 % of HGPIN lesions in proximity to cancer are also positive for ERG rearrangement with identical ERG gene fusions. Evaluation of ERG oncoprotein expression in whole mount sections using clone 9FY has revealed a strong concordance between focally ERG-positive HGPIN and homogenously ERG-positive prostate cancer in 96.5 % of cases [31]. These findings indicate a clonal relationship between gene fusion-positive HGPIN and cancer and thus potential implications for utilization of ERG as a marker for prostate cancer risk stratification in patients with HGPIN diagnosis. Two studies examined the significance of ERG-positive HGPIN for future cancer risk stratification and came to contrasting conclusions. Gao et al. found that the presence of ERG rearrangement in HGPIN lesions detected on initial biopsy warrants repeat biopsy [36]. He et al., however, did not find the utility of ERG to stratify cancer risk associated with HGPIN [37]. Patients with initial HGPIN in biopsies and at least one follow-up prostate biopsy were included and were immunostained for ERG. The cancer detection rate was not significantly different between ERG-positive and ERG-negative HGPIN cases. The authors concluded that ERG expression is distinctly uncommon in isolated HGPIN (5.3 %) and positive ERG expression is not associated with increased cancer detection in subsequent repeat biopsies [37].
A repeat prostate biopsy after 3–6 months is recommended for patients with ATYP diagnosis due to its high predictive value for cancer detection in repeat biopsy. In a study of follow-up biopsies from 103 patients with a preliminary diagnosis of ATYP, ERG expression was detected in 16 cases (15.5 %). Of these 16 ERG-positive cases, the atypical glands were positive for ERG in nine [34]. Five of these patients (55.6 %) had cancer on repeat biopsies, compared with 42 of the 87 (48.3 %) with ERG-negative preliminary biopsies. The authors concluded that ERG expression is unlikely to help identify patients suitable for subsequent biopsies. Of note, in the study the repeat biopsies were not directed to the ERG-positive ATYP sites. Overall, additional biopsy studies assessing ERG-positive HGPIN and ATYP lesions are needed to evaluate more thoroughly the utility of measuring ERG expression as prostate cancer risk stratification in subsequent biopsies in patients with HGPIN or ATYP.
Utility of ERG in the Workup of Atypical Cribriform Lesions on Prostate Needle Biopsy
Atypical cribriform lesions of the prostate gland are defined as cribriform or rarely solid prostate glands populated by cytologically malignant cells with preservation of basal cells. It may represent cribriform HGPIN or intraductal carcinoma of the prostate (IDC-P) [38]. IDC-P is almost always associated with high-grade and high-volume invasive carcinoma. On the other hand, cribriform HGPIN is a putative neoplastic precursor lesion; recent studies have shown that the significance of HGPIN as a predictive marker of cancer has reduced significantly in the range of 25 % [39, 40]. The diagnosis of focal HGPIN, defined by < 2 cores involvement, currently does not mandate a repeat biopsy within the first year of diagnosis [39]. In contrast, prostate cancer with associated IDC-P component has a significantly worse prognosis than cancer without IDC-P [40]. Therefore, the distinction of cribriform PIN from IDC-P is of paramount importance due to its widely differing clinical significance. In a study evaluating ERG gene fusions in a subset of cribriform HGPIN (noncancer-associated atypical cribriform lesions) and IDC-P lesions (cancer-associated atypical cribriform lesions) in totally embedded radical prostatectomy specimens, isolated cribriform HGPIN lesions consistently lacked ERG gene rearrangements, while IDC-P lesions regardless of their morphologic spectrum were highly enriched in these gene fusions [38]. ERG gene rearrangements were observed in up to 75 % of IDC-P. Therefore, all cancer-associated atypical cribriform lesions essentially represent an intraductal spread of prostate cancer, and ERG IHC has potential utility in stratification of an atypical cribriform lesion encountered in prostate biopsy. Essentially all ERG expressing atypical cribriform lesions, especially when associated with adjacent prostate cancer represent examples of IDC-P.
Utility of ERG IHC in the Evaluation of Metastatic Tumor of Unknown Origin
ERG is known to be expressed in endothelial cells, and oncogenic ERG gene fusions occur in subsets of prostatic carcinoma, acute myeloid leukemia, and Ewing sarcoma [41, 42]. In vascular tumors, ERG oncoprotein is expressed in a broad range of tumors including hemangiomas, lymphangiomas, angiosarcomas, epithelioid hemangioendotheliomas, and Kaposi sarcomas. Among nonvascular mesenchymal tumors, Ewing sarcoma and blastic extramedullary myeloid tumors express ERG. Among epithelial tumors, diffuse ERG expression is largely restricted to prostatic adenocarcinomas. Rare other carcinomas and epithelial tumors that may demonstrate focal ERG expression include large cell undifferentiated pulmonary carcinomas, mesotheliomas, thymomas, squamous cell carcinomas of the skin and lung, carcinosarcomas of the uterus, gastrointestinal stromal tumors, hepatocellular carcinomas, teratomas of the testis, anaplastic carcinomas of the thyroid, giant cell tumors of the tendon sheath, and benign fibrous histiocytomas of the skin [43]. Overall, ERG has a very narrow biological role in highly selected tissues and among epithelial tumors in appropriate clinical setting, strong and diffuse ERG expression would essentially support the diagnosis of prostate carcinoma [25]. Similarly, in the setting of small cell carcinoma of unknown origin, ERG positivity would support the prostatic origin of small cell carcinoma [44, 45].
Limitations of ERG IHC as Diagnostic Biomarker
Despite several promising clinical applications, ERG has several important limitations as a diagnostic prostate cancer biomarker that needs to be addressed. While positive ERG establishes a diagnosis of prostate cancer in the majority of cases, negative ERG expression offers no value in the work up of atypical cases as ERG overall has a low sensitivity for prostate cancer detection [23]. In addition, ERG expression should be interpreted with caution if the small atypical glands are either intermingled or closely associated with HGPIN glands (PINATYP) [23, 24]. As ERG is expressed in small proportion of HGPIN glands, a diagnosis of HGPIN or PINATYP cannot be ruled out with certainty in such cases. Whether ERG protein expression in such situations is a marker of unsampled adjacent cancer still remains to be addressed. Similarly, inter-focal tumor heterogeneity for ERG expression observed within multifocal prostate cancer may also potentially affect the utilization of ERG as a diagnostic, prognostic, or predictive prostate cancer biomarker.
A summary of biology, clinical applications, and pitfalls of ERG oncoprotein is summarized in Table 9.4.
Table 9.4
ERG protein
Genetics |
ERG is a member of the ETS gene family, which is commonly (~ 50 %) involved by chromosomal translocation in prostate cancer |
Fusions between TMRSS2 and ERG represent the most common molecular subtype accounting for ~ 90 % of ETS gene fusions |
Staining pattern |
Nuclear staining |
ERG immunostaining correlates highly with ERG gene alteration |
Endothelial cells are strongly positive for ERG and serve as the positive internal control |
Clinical utility |
In small atypical glands where the diagnosis of HGPIN is ruled out, positive staining supports the cancer diagnosis |
In small proportion of cases, positive ERG may help convert an ATYP diagnosis to cancer |
ERG expression in atypical cribriform lesion (containing basal cells) supports the diagnosis of intraductal carcinoma of the prostate, specifically when associated with adjacent invasive carcinoma |
Diffuse ERG expression in metastatic carcinoma of unknown origin supports the prostatic origin of carcinoma |
Measurement of ERG overexpression in conjunction with PTEN loss may improve prostate cancer risk stratification |
Pitfalls |
Low sensitivity for prostate cancer detection; positive in 40–50 % of prostate carcinomas |
Positive in 20 % of HGPIN that intermingles with prostate carcinoma |
ERG expression may demonstrate frequent inter-focal tumor heterogeneity |
Glutathione s-Transferase π 1
Biology
Glutathione s-transferase π 1 ( GSTP1) gene methylation is the most common epigenetic change in prostate cancer [46]. Methylation silences the gene depriving normal cells of protection against damage by oxidation and electrophilic substances and subsequent malignant transformation [46]. GSTP1 expression is rarely detected in prostate cancers. Methylation of the GSTP1 gene is present in PIN and cancer but not in benign glands .
Clinical Applications
Using methylation-specific polymerase chain reaction (PCR) assay, detection of the methylated GSTP1 promoter region is utilized as a tissue-based diagnostic marker to differentiate PIN and cancer from benign prostate tissue including BPH. Absent or decreased GSTP1 activity in cancerous tissue has been suggested as a potential prognostic marker [47].
Urine-Based Molecular Markers of Diagnosis
Prostate Cancer Antigen-3
Biology
First identified in 1999, initially known as DD3 gene and later called prostate cancer antigen-3 ( PCA3) , is overexpressed in more than 95 % of all prostate cancers with high prostate specificity. The PCA3 gene is located at 9q21–22, and encodes a nontranslational transcript [48–50]. PCA3 assays have been developed using ribonucleic acid (RNA) detection methods since no protein products have been detected from PCA3 RNA.
Clinical Applications
Urinary test, to detect PCA3, is performed following thorough digital rectal examination (with three strokes on each lobe). In the first voided urine sample, PCA3 and PSA RNAs are selected, amplified by transcription-mediated amplification and detected by hybridization protection assay. PCA3 score is calculated as PCA3/PSA ratio multiplied by 1000. The test is considered positive when the PCA3/PSA ratio is equal to or greater than 35. Clinical studies have demonstrated the sensitivity of the PCA3 test (range: 54–82 %) to be less than serum PSA, whereas the specificity of PCA3 (66–89 %) to be better. The positive predictive values (48–75 %) and negative predictive values (74–90 %) for PCA3 are also better than for PSA. The accuracy of the urinary PCA3 test ranges from 66 to 84 % [51]. Patient’s age, inflammation, trauma, 5 α-reductase inhibitor use, or prostate volume do not significantly influence the test [52].
PCA3 test has been FDA approved for its ability to predict cancer in patients with increased PSA and negative biopsy (Fig. 9.5). Additionally, it has also shown utility in refining prostate cancer risk in men undergoing initial prostate biopsy (most commonly due to elevated serum PSA) [53].
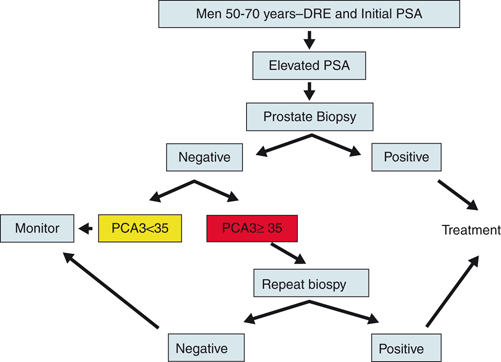

Fig. 9.5
Flow chart demonstrating current clinical applications of urine PCA3 test. PSA prostate-specific antigen, DRE Digital rectal examination
Patients with atypical small acinar proliferation and high-grade prostatic intraepithelial neoplasm have a higher mean PCA3 score as compared to patients with noncancerous prostate. The mean score, however, is significantly lower than for patients with a definitive diagnosis of prostate cancer [51].
There have been conflicting reports regarding the usefulness of PCA3 in active surveillance of prostate cancer and is undergoing further evaluation. A recent study incorporated PCA3 in the management of prostate cancer . PCA3 score combined with traditional tools may aid in identifying men with clinically insignificant disease who would be candidates for active surveillance. A low PCA3 score (of 20) may have the highest utility for selecting men with clinically insignificant prostate cancer in whom active surveillance may be appropriate; a higher PCA3 score (of 50) may be useful to identify men at higher risk of harboring significant prostate cancer who would be candidates for radical prostatectomy [53].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


