Stage
Flocks and Kadesky—1958
Petkovic—1959
Robson—1969
5-year survival (%)
5-year survival (%)
5-year survival (%)
1
Limited to renal capsule
55
Renal limited and encapsulated
75
Renal limited
66
2
Renal pedicle ± fat invasion
40.5
Renal limited with capsular invasion
55
Perirenal fat but within Gerota’s fascia
64
3
Regional lymph nodes
9.5
Extrarenal into fat, veins, lymphatics
4
3a. Gross renal vein or inferior Vena Cava (VC)
3b. Lymphatics
3c. Veins and lymphatics
42
4
Distant metastasis
3.5
Distant metastasis
6
4a adjacent organs
11
4b distant metastasis
The second staging system was formulated by Petkovic a year later (Table 24.1) [5]. Curiously, he did not cite the Flocks and Kadesky paper even though they were both published in The Journal of Urology. In contrast to Flocks and Kadesky’s staging system, Petkovic’s staging system had two renal-limited categories, one category for local spread and one for distant metastases. Petkovic provided drawings to illustrate his tumor stages . Use of such visual aids is helpful to pathologist and surgeon alike, a practice currently followed by TNM staging. Petkovic commented that tumor growth characteristics are more important than tumor size, and noted that even patients with apparently renal-limited tumors may have metastases, a vexing problem with all staging systems.
The third staging system was formulated by Robson in 1969 (Table 24.1) [6]. The Robson’s system was similar to Flocks and Kadesky having a single renal-limited stage and two stage designations for local extension. Robson emphasized the additional prognostic factor of tumor grade in addition to stage. This system was popular well into the 1980s despite the existence of the TNM staging system for more than a decade, possibly because the Robson system was featured in the first Armed Forces Institute of Pathology (AFIP) fascicle.
The Tumor-Node-Metastasis (TNM) Classification
The fourth and internationally utilized staging system is the Tumor-Node-Metastasis (TNM) Classification of Malignant Tumors. The TNM system was developed between 1943 and 1952 by Pierre Denoix, a French surgeon [3] . The International Union Against Cancer (UICC) published ten manuals between 1956 and 1967 that incorporated his TNM staging recommendations for tumors of two dozen body sites. In 1968, these were collated into the first edition of the TNM Classification. Kidney was one organ, however, that was not included in this initial formulation. It did appear in the second edition published in 1974. The initial inclusion of kidney almost seems like an afterthought based upon the definitions of the pT1 and pT2 categories in both the 1974 TNM and the 1978 TNM editions which were listed as “small” and “large” [7]. In 1959, the American Joint Commission on Cancer (AJCC) was founded. Since the 1980s, the UICC and AJCC have worked together and simultaneously published the TNM Classification of Malignant Tumors by the UICC, and the Cancer Staging Manual by the AJCC . The revision cycle is 6–8 years. From 1968 to 2009, seven iterations of the TNM Classification have appeared; an eighth iteration can be expected soon [3, 7–9].
The TNM system classifies extent of disease based on anatomic information about the primary tumor (pT stage), regional lymph nodes (pN stage) and metastases (pM stage) [3, 9]. These are combined into four stage groups. These can be purely clinical groupings, pathological groupings, or a combination of data may be employed. The latter is common in RCC since regional lymph node dissections are infrequently performed. The complete 2009 TNM pT, pN, and pM stage definitions and the stage groupings are provided at the end of this chapter .
Tables 24.2 and 24.3 list the staging parameters for most of the TNM formulations so that the evolution of TNM for RCC can be appreciated. The recurrent themes of tumor size stratification, the definitions of local extension, and the optimum handling of vena cava (VC) invasion represent the ongoing attempts to optimize stage prognostication, struggles inherent to the evolutionary process that characterizes TNM staging. The evolution of stage definition is important to keep in mind when comparing stage-related outcome data over time because a change in stage definition affects the tumor composition of the stage groupings. A notable example was the major change in tumor size criteria for pT1 versus pT2 between the 1987 TNM and 1997 TNM when the pT1/2 break point was shifted from 2.5 to 7 cm. This drastically increased the proportion of stage I tumors and reduced the proportion of stage II tumors .
Table 24.2
Evolving definition of the TNM renal cell carcinoma staging system
UICC/AJCC | pT1 | pT2 | |||
|---|---|---|---|---|---|
1968 TNM excluded the kidney | |||||
1978 | Small, without enlargement of kidney, limited distortion pelvis, calyces or vessels | Large, with enlargement of kidney, or pelvicalyceal involvement | |||
1987 | < 2.5 cm renal limited | > 2.5 cm renal limited | |||
1997 | < 7.0 cm renal limited | > 7.0 cm renal limited | |||
pT1a | pT1b | pT2 | |||
2002 | 4 cm or less renal limited | > 4–7.0 cm renal limited | > 7.0 cm renal limited | ||
pT2a | pT2b | ||||
2009 | 4 cm or less renal limited | > 4.7.0 cm renal limited | > 7–10 cm renal limited | > 10 cm renal limited | |
Table 24.3
Evolving definition of the TNM renal cell carcinoma staging system
UICC/AJCC | pT3 | pT4 | |||
|---|---|---|---|---|---|
1968 excluded kidney | |||||
1978 | Involvement of perinephric fat or hilar vessels | Involvement of neighboring organs or abdominal wall | |||
pT3a | pT3b | pT3c | pT4a | pT4b | |
1987 | Perinephric fat or adrenal | RV involvement | VC below diaphragm | Beyond Gerota’s fascia | VC above diaphragm |
pT4 | |||||
1997 | Perinephric fat or adrenal | RV or IVC below diaphragm | VC above diaphragm | Beyond Gerota’s fascia | |
2002 | Perinephric fat includes sinus fat or adrenal | RV includes muscular sinus veins or VC below diaphragm | VC above diaphragm | Beyond Gerota’s fascia | |
2009 | Gross involvement of RV or segmental muscle containing branches, perinephric and/or sinus fat | Gross extension into VC below diaphragm | Gross extension into VC above diaphragm or invades VC wall | Beyond Gerota’s fascia, or contiguous extension into adrenal gland | |
Several notable modifications appeared in the 2002 and 2009 TNM formulations relating to the size and local extension Tables 24.2 and 24.3. Substages for the pT1 and pT2 categories were introduced in the 2002 and 2009 TNMs, respectively. However, possibly the most significant modification occurred in the 2002 TNM with incorporation of renal sinus invasion into the pT3 category [9]. This mandated a paradigm shift in specimen handling.
Additional important modifications in 2009 TNM recognized the importance of direct adrenal extension, and added VC wall invasion.
In 2012, the International Society of Urological Pathology (ISUP) convened a Consensus Conference at the US and Canadian Academy of Pathology annual meeting. Over 130 urologic pathologists from around the world participated. This was preceded by a comprehensive survey that included queries relating to specimen handling and TNM staging, and many other topics. The survey documented diagnostic criteria and practice behaviors while the Consensus Conference led to practice recommendations based upon achieving a 65 % consensus threshold among participants [10]. Issues failing to achieve consensus provided topics for future investigation and possible subsequent incorporation into the next TNM formulation .
pT1 and pT2 Substages
The prognostic relationship between size and outcome has been known for almost a century. In 1937, ET Bell, MD, published a seminal study of 71 RCCs in which he found that only one of 38 tumors less than 3 cm developed metastases [11]. This subsequently led to the long-lived, but now defunct 3 cm rule, used to distinguish so-called adenomas from carcinomas. Recently, the Mayo Clinic Group examined a much larger number of patients and found an even lower incidence of metastases; only one of 781 patients with M1 disease had an RCC 3 cm or less [12].
Dr. Bell also found that with increasing tumor size the metastatic rate increased, findings repeatedly confirmed. Multiple large series of RCC have demonstrated that tumor size is an independent predictor of cancer-specific survival, risk of metastases at presentation, recurrence rates, and patient outcomes [7, 13–15]. However, for size to retain its prognostic importance size determination must not only be accurate but measurements must be made in a standard fashion. The ISUP Consensus Conference recommends that size be determined following multiple parallel sections through the tumor [10]. The primary tumor measurement should include perinephric fat extension , both peripheral perinephric fat and central sinus fat. Satellite nodules, renal vein, and VC involvement, however, should not be included.
The introduction of the first size subgroup of renal-limited RCC involved pT1 tumors and appeared in 2002 TNM [8]. It divided pT1 into pT1a, defined as RCC 4 cm or less, and pT1b, defined as RCC > 4–7 cm. This 4 cm cutoff was originally introduced to identify a group of tumors suitable for nephron sparing surgery, a procedure initially heavily weighted toward tumors 4 cm or less. However, this clinical relevance is diminishing since much larger tumors are now similarly treated. Although multiple large studies of RCC have noted a difference in patient outcomes with breakpoints in the 4–5.5 cm size range validating a pT1 subgrouping, Delahunt et al. suggest that size may actually represent a continuous variable [14]. They have shown a 3.51x risk of cancer-related death for each doubling in tumor size.
The second size subgroup introduced affected pT2 tumors and appeared in the 2009 TNM [9]. Its implementation was based primarily upon a study from the Mayo Clinic of 544 patients treated for RCC (77.7 % clear cell type) [15]. They found a survival difference for tumors “renal limited” in the 7–10 cm versus “renal-limited” tumors > 10 cm. Stage pT2 tumors are now stratified in 2009 TNM into pT2a, defined as renal-limited tumors > 7–10 cm, and pT2b, defined as renal-limited tumors > 10 cm. More recently, however, Waalkes et al. in a validation study of 2009 TNM restaged 5122 patients with pT2 tumors and found no difference in cancer-specific survival for pT2a versus pT2b [16]. Novara et al. in a recent European multi-institutional validation study of 2009 TNM that included 5339 patients, found excellent stratification in 5- and 10-year cancer-specific survival for most stages [17]. However, both pT1a and pT1b, and pT2b and pT3a had similar Cancer specific survival (CSS) (Fig. 24.1). These data raise concern that substage definitions may be somewhat arbitrary and that large tumor size may be a surrogate marker for undetected extrarenal spread, especially invasion of the renal sinus. The 7 cm cutoff for pT2 tumors may have another unintended consequence, the selection of a more indolent subset of RCCs enriched for certain RCC types. Using the Surveillance Epidemiology and End Results (SEER) data, Rothman et al. found that 70 % of pT2 tumors over 7 cm were not only low grade, they were overrepresented for the more indolent RCC types of papillary RCC and chromophobe cell RCC [18].
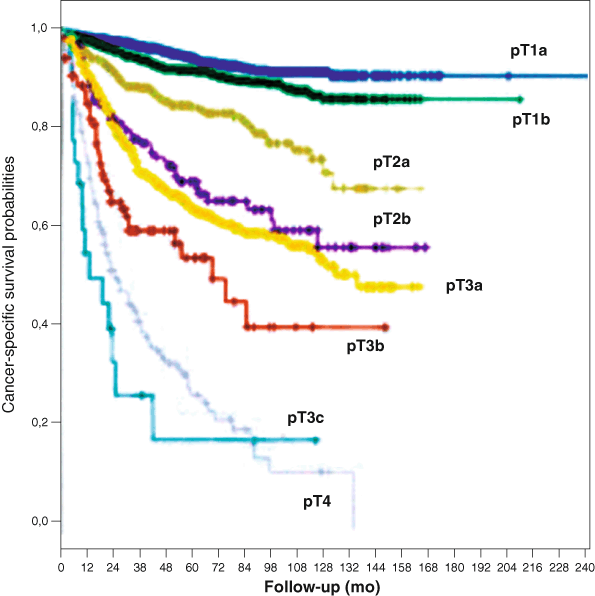

Fig. 24.1
Cancer-specific survival (CSS) probability according to the 2009 TNM staging system (log-rank pooled over strata p< 0.0001). Five-year CSS was 94.9 % in pT1a ( blue curve), 92.6 in pT1b ( green curve), 85.4 % in pT2a ( gray curve), 70 % in pT2b ( violet curve), 64.7 % in pT3a ( yellow curve), 54.7 % in pT3b ( red curve), and 27.1 % in pT4 ( lightgray curve). All the pairwise survival differences among the different pT stages were statistically significant with the exception of those observed between pT2b and pT3a cancers (log-rank pairwise p = 34) and between pT3c and pT4 cancers (log-rank pairwise p = 26). (Used with permission from [17])
pT3a Regional Extension
Robson stated in 1969 “…the hope for a cure lies in the hands of the surgeon” [6]. However, the hope for accurate tumor prognostication lies in the hands of the pathologist. A lofty goal for a RCC staging system, and possibly the “holy grail” for a specimen examination protocol, is to stratify those cases in which a surgical cure can be expected from those cases with risk of residual disease. Renal-limited tumors, pT1 and pT2, represent the only stage categories in which a surgical cure is feasible. Regrettably, every staging system, from Flocks and Kadesky to the 2009 TNM, fall short of predicting surgical cures. The National Cancer Data Base (NCDB) indicates that for 37,166 tumors resected for years 2001 and 2002, there was an 81 and 74 % observed survival (cancer deaths plus death due to other causes) for stage I and stage II RCC, respectively (Fig. 24.2) [9]. Many stage I and II patients whose deaths are attributable to metastatic disease had RCCs that were not renal limited at the time of nephrectomy.
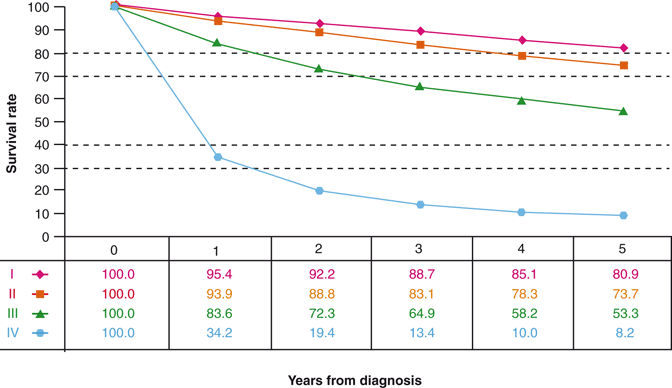

Fig. 24.2
Observed survival rates for 37,166 patients with kidney cancer classified by the 2009 AJCC staging classification. Data taken from the National Cancer Data Base (Commission on Cancer of the American College of Surgeons and the American Cancer Society) for the years 2001–2002. Stage I includes 18,912 patients; stage II 4443; stage III 5952; and stage IV 7859. (Used with permission from [9])
In 1969, the National Wilms Tumor Study (NWTS) was initiated in the hope of identifying the optimal therapy for Wilms tumor. From the onset of the NWTS, there was standardized specimen handling and central review of the nephrectomy findings by Bruce Beckwith, MD. One of Dr. Beckwith’s many contributions to the understanding of Wilms tumor and other pediatric renal neoplasms was the recognition that the renal sinus was the principal metastatic pathway [19]. This seems now intuitive since the majority of the renal parenchymal venous and lymphatic outflow pass through the renal sinus as detailed in Chap. 22. Initially, the NWTS threshold for upstaging from renal-limited stage I to extrarenal stage II was extension of tumor beyond the “hilar plane.” This was a difficult judgment to make for the primary prosector, and even more challenging to validate on central review. In the NWTS-5, the fifth clinical trial launched in 1995, involvement of sinus vessels or extensive sinus fat involvement qualified for stage 2 designation. At that time, a similar role of the renal sinus involvement in RCC had not been investigated.
In 1998, I initiated a study of renal sinus involvement in RCC by totally embedding the interface between the renal sinus and the RCC. The intent of the study was to determine if pT1 and pT2 tumors staged by the 1997 TNM were truly renal limited or if some tumors extended into the renal sinus which by definition is outside of the kidney. The results of a small series of 31 cases of RCC reported in 2000 found that 14 of 31 (45 %) cases had invaded the renal sinus fat and/or sinus veins [20]. Most significantly, seven of 14 cases of stage pT1 and pT2 RCC by 1997 TNM criteria were not, in fact, renal limited. They had not only extended into the renal sinus but also into sinus veins.
In 2002, TNM renal sinus fat and renal sinus “muscular” vein involvement were incorporated into pT3a and pT3b, respectively [8]. Sinus invasive disease was further modified in 2009 TNM; “gross” involvement of renal vein or “segmental muscle containing” (sinus) branches were combined with sinus fat invasion into pT3a [9]. These modifications moved extrarenal sinus invasive disease included with renal-limited pT1 and pT2 categories prior to 2002, to the extrarenal pT3 category. This advance should improve stage-related prognostication. It should decrease the slope of the survival curves for stage I and stage II RCC, as illustrated in Fig. 24.2 by eliminating a group of extrarenal disease that contaminated stage groups I and II. It could also contribute to the stage migration that has occurred due to the increased incidence of small incidentally discovered pT1 tumors [21]. More accurate RCC staging relative to the renal sinus could also shift a number of RCCs from the pT2 category into the pT3 category, making pT2 RCC, and particularly pT2b, a less common tumor.
It is important to appreciate that most studies of outcome relative to stage have utilized large archival data bases that include many cases accessioned prior to 2002, often dating back to the 1970s. A significant percentage of the pT1 and pT2 tumors staged by 1997 TNM and earlier formulations and included in the NCDB and other data bases, may not have been renal limited. They are likely under-staged pT3 cases. These cases could account for a substantial fraction of the 15–20 % metastatic disease that develops in stage I and II RCC. This is not a criticism of those studies, but simply a reflection that the renal sinus was not sampled since its importance had not yet been appreciated. Evidence in support of this contention was provided by Thompson et al. in 2007 [22]. They reexamined the nephrectomy specimens of 33 cases of stage I RCC by 1997 TNM criteria who died from metastatic RCC. They found that 67 % of the RCCs had extended into the renal sinus fat or sinus veins, and were not, therefore, renal limited. They were under-staged pT3 tumors by 2002 and 2009 TNM criteria. Now that the renal sinus invasion has been part of the TNM staging system for over 10 years, outcomes studies should limit their cases to those accessioned after 2002 at the time when renal sinus examination was incorporated into their specimen handling protocol.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


