INTRODUCTION
REVIEW OF NORMAL HISTOLOGY OF THE LOWER URINARY TRACT “QUICK REVIEW”
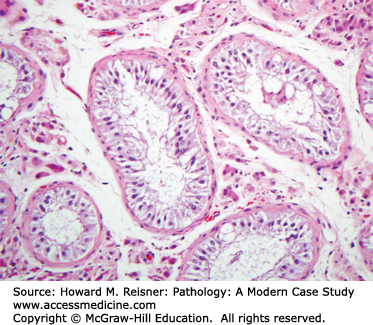
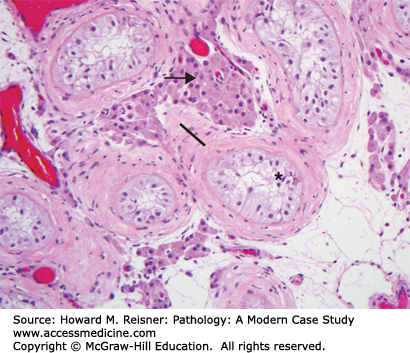
The outflow of the kidneys (renal pelvis, ureters, bladder, and urethra) comprises the lower urinary tract (Figure 13-1). The basic histology is that all are lined by urothelium (transitional epithelium), which covers a connective tissue layer of lamina propria, a variably thick layer of smooth muscle (muscularis propria) and adventitia (Figure 13-2). Urothelium is specialized epithelium that is multilayered with large surface superficial cells (umbrella cells), which can change shape as the bladder dilates and contracts. These contain specialized cell junctions that are resistant to permeation by urine. Beneath this are five to six layers of smaller urothelial cells. The muscularis propria is well developed in the bladder, and coordinated contraction of this muscle (detrusor muscle) helps in emptying the bladder. Muscle is less prominent in the ureters and the urethra. The distal urethra is lined by squamous epithelium, which is continuity with the squamous epithelium of the external genitalia. Both the male and female urethra contain mucinous glands in their walls.
REVIEW OF LOWER URINARY TRACT AND NORMAL MALE GU DEVELOPMENT
The lower urinary tract develops from the anterior part of the cloaca. In early fetal development, this area of the cloaca becomes the urogenital sinus, and develops into the fetal urinary bladder, proximal urethra, and the urachus. The distal urethra develops from the urogenital membrane, and the proximal and distal structures fuse. The urachus is a temporary structure that connects to the umbilicus, and this involutes in later fetal life. The ureters develop as lateral buds from the bladder, grow cranially, and induce the formation of the kidney from the metanephric blastema.
Development of the male genital system is complex. The testes begin as intra-abdominal organs, from the genital ridges in the coelomic cavity. These contain primordial germ cells that are originally from the yolk sac and migrate into the genital ridges. Testes develop from this mixture of tissues, and they migrate from their intra-abdominal location into the inguinal canal and eventually the scrotum. During development in the abdomen, testes connect with their outflow tracts derived from Wolffian ducts. These develop into the epididymis and vas deferens. The external male genitalia develop from the genital tubercle and the anterior urogenital sinus. The presence of testosterone allows development of these primordial tissues into the scrotum and penis.
URINARY BLADDER
Principal diseases of the bladder include congenital anomalies, inflammatory conditions, benign proliferative lesions, and tumors. Obstructive disease of the bladder is most commonly related to prostatic hypertrophy and will be discussed with the prostate.
The bladder is complex embryologically, and thus congenital anomalies are not uncommon.
Exstrophy of the bladder refers to absence of the anterior wall of the bladder (due to incomplete resorption of the anterior cloacal membrane), and in this condition the bladder is open on the abdominal skin. Urine drains freely out of the defect, and the open posterior bladder wall quickly becomes inflamed and undergoes squamous and glandular metaplasia. This condition is now currently corrected surgically early in life, but in past times patients with uncorrected exstrophy had a high risk of malignant transformation related to constant inflammation and irritation of epithelium. However, even patients with corrected exstrophy are of increased risk because of the metaplasias present in the residual urothelium.
Diverticula of the bladder are due to outpouchings of the urothelium and lamina propria through areas of weakness in the muscle layers, and the urothelium within these diverticula may become inflamed and stones may form.
Urachal remnants are persistent glandular structures from the fetal urachus, and may form fistula, cysts, or tumors. The tumors that arise from these remnants are usually adenocarcinomas.
Cystitis is inflammation of the bladder. It may be acute, chronic, or granulomatous, infectious or noninfectious. Risk factors include (1) being of female sex (because females have shorter urethras than males), (2) having urinary outflow obstruction causing stagnation of the urine (most commonly a result of benign prostatic hyperplasia (BPH) in males), (3) history of instrumentation of the bladder (such as catheterization or cystoscopy), (4) presence of bladder stones, and (5) history of diabetes, immunodeficiency, radiation, or chemotherapy. Coliforms are the most common bacterial pathogen. Worldwide, tuberculous cystitis is common and is secondary to renal tuberculosis.
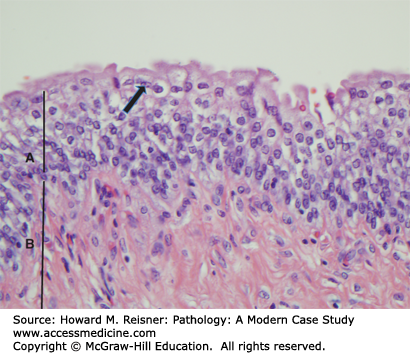
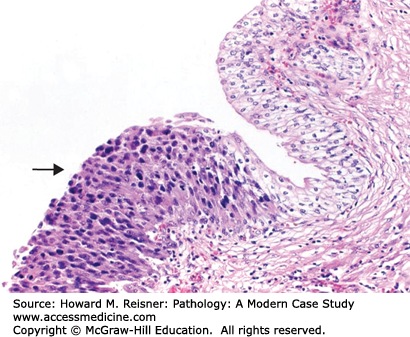
Acute cystitis (Figure 13-3) shows acute inflammatory cells (neutrophils), edema, and hemorrhage of the epithelium and lamina propria (Figure 13-4). Chronic cystitis shows an infiltrate with lymphocytes and plasma cells, and if long-standing fibrosis of the lamina propria. The chronic inflammatory cells may organize into lymphoid follicles with germinal centers (follicular cystitis). Granulomatous cystitis shows epithelioid macrophages and giant cells. This may be seen with tuberculosis and other mycobacteria, such as Bacillus Calmette–Guerin, which is instilled into the bladder as a treatment of some bladder cancers.
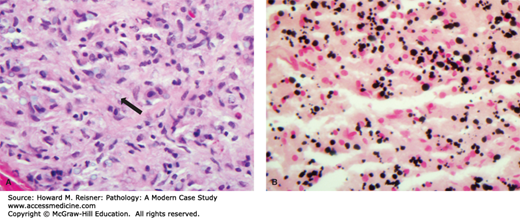
Malakoplakia is a rare inflammatory disorder identified by the accumulation of foamy macrophages. The aggregation of macrophages is often seen grossly as a yellow plaque or mass. The macrophages contain variable numbers of calcified, laminated inclusions that are known as Michaelis–Gutmann bodies (Figure 13-5 see case below). These structures are lysosomes that contain partially degraded bacteria. Malakoplakia is thought to be a disorder associated with defective bacterial destruction by macrophages. It is most often seen in immunosuppressed patients infected with Escherichia coli, although other bacteria may be implicated. The incompletely degraded bacteria and engorged lysosomes serve as a nidus of calcification. The most common site of malakoplakia is the bladder, although other sites both within and outside of the urinary tract may be involved.
FIGURE 13-5
Photomicrograph of malakoplakia (A) The section stained with H&E shows poorly defined lucent inclusions within cells (arrow). (B) The section has been prepared using the von Kossa stain that highlights calcium deposits in black. The dark cellular inclusions represent Michaelis–Gutmann bodies, lysosomes engorged with partially degraded, and calcified bacteria.
HUNTING FOR ZEBRAS
Schistosomiasis is a parasite endemic in North Africa and the Middle East. The organism enters through the skin, often in the lower leg, through contact with infected water. It causes marked chronic inflammation of the bladder wall secondary to organisms that settle in the veins of the bladder and provoke an intense inflammatory response. As a result the urothelium undergoes squamous metaplasia. Patients with long-standing schistosomiasis are at increased risk of malignancy, which are usually squamous cell carcinomas.
Benign proliferative and metaplastic lesions are common in the urinary tract. These are often associated with inflammation, but can also be seen with chronic irritation such as stones or an indwelling catheter. Bladder exstrophy is often associated with widespread proliferative lesions.
Brunn nests and Brunn buds are invaginations of the surface epithelium that may be either detached (nests) or attached (buds) to the surface. These are so common as to be variants of normal.
Cystitis cystica is cystic change within Brunn nests and buds. These may be seen grossly as clustered tiny cysts on the bladder surface. Glandular metaplasia to mucinous epithelium may occur within the epithelium of cystitis cystica, and this is termed cystitis glandularis.
Squamous metaplasia is transformation of the normal urothelium to squamous epithelium. It is common in the trigone of adult women and in this location is probably a variant of normal. It is less common in men, and is more likely to be associated chronic irritation and inflammation.
Nephrogenic metaplasia (nephrogenic adenoma) is a metaplastic change of urothelium to renal tubular type epithelium. It may produce clusters of tubules in the lamina propria or an exophytic papillary mass.
CASE 13-1Malakoplakia
A 52-year-old lung transplant patient complains of urinary tract symptoms of urgency and frequency. Cystoscopic examination of the bladder shows a 2 cm yellow plaque-like mass in the dome of the bladder. Biopsy of this mass (Figure 13-5) shows numerous macrophages with abundant granular cytoplasm with a sprinkling of neutrophils and lymphocytes. The macrophages are positive with periodic acid–Schiff stain. Some of the macrophages contain laminated basophilic structures that are positive with von Kossa stain for calcium.
Patients with extensive metaplasia and long-standing inflammation are at increased risk of the development of malignancies, and the malignancies that arise in these settings may be of the metaplastic tissue type. Thus, patients with extensive squamous metaplasia are at risk of development of squamous cell carcinoma (such as patients with schistosomiasis) and those with extensive cystitis glandularis are at risk of adenocarcinoma (such as is found in association with exstrophy).
Chronic obstruction of the bladder (such as by BPH) causes changes in the bladder including dilatation, muscle hypertrophy, trabeculation, and diverticula. Patients are often symptomatic with lower urinary tract symptoms (LUTS). This is discussed further in the section on benign prostate hyperplasia.
CASE 13-2Bladder Cancer Linked to Cytoxan (Cyclophosphamide) Exposure
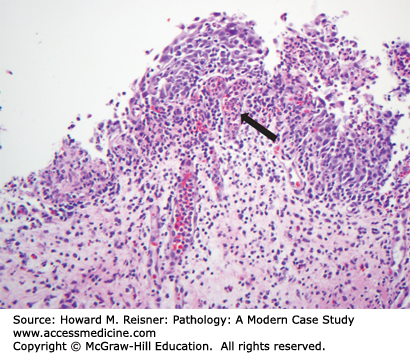
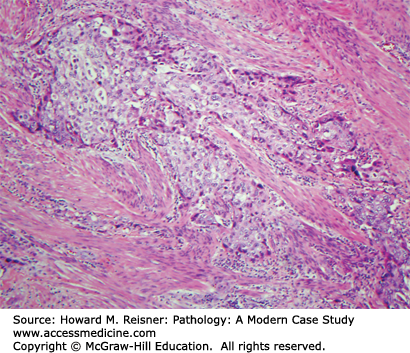
A 40-year-old female visits her family practice physician with a complaint of gross hematuria. Her past medical history is significant for Hodgkin disease treated with chemotherapy (including Cytoxan) when she was a teenager. Urine culture is taken but is negative, but nevertheless the patient is given a course of antibiotics. The antibiotics do not clear the hematuria and the patient is referred to a urologist. Bladder cytology shows malignant cells (Figure 13-6), and cystoscopy shows a ragged tumor involving the left bladder sidewall (Figure 13-7). Biopsy of this tumor shows invasive high-grade urothelial carcinoma invasive into the muscularis propria (Figure 13-8).
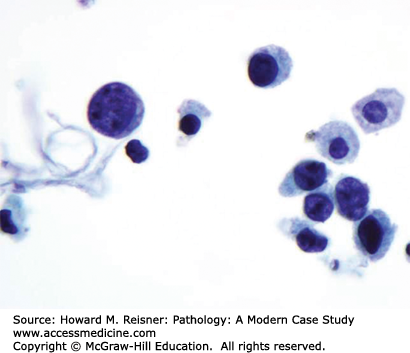
FIGURE 13-6
Urine cytology with malignant cells. Factors suggesting malignant urothelium include the following. Cells have markedly increased nuclear to cytoplasmic ratios with hyperchromatic nuclei. Intracytoplasmic vacuoles are common. Several cells are spindle shaped. Cells are discohesive. Abnormal cells are numerous
The most common site of tumors of the urinary tract is the bladder. Bladder cancer is at least partially related to carcinogens present in the urine, and for that reason all of the urothelium (i.e., all cells bathed by urine) is at risk of development of malignancy in a patient with a urothelial tumor (resulting in the so-called field effect of urothelial malignancy). The bladder is the most frequently involved site, because (1) the majority of the urothelium is found in the bladder, (2) the bladder is the storage organ for urine, and hence (3) the bladder urothelium has the most intense exposure to any urinary carcinogens. Known carcinogens associated with urothelial tumors include benzene dyes (arylamines), some chemotherapeutic agents (such as Cytoxan mentioned above), analgesics that are excreted in the urine, and cigarette smoking (since smoking related carcinogens are excreted in the urine). Historically, arylamines were the most common agent associated with bladder cancer. The concept of carcinogens in the urine being associated with urothelial cancer was first defined in textile workers in New England. Workers involved with the dyeing of cloth had an extraordinarily high incidence of bladder cancer in the late 19th and early 20th century, before environmental controls were in place. Currently, cigarette smoking is the most common risk factor for bladder cancer. Schistosomiasis is associated with bladder cancers, because of the chronic inflammation and squamous metaplasia associated with long-standing infection with this organism. A large number of chromosomal abnormalities have been identified in bladder cancer.
IN TRANSLATION Molecular Based Screening Tests for Urothelial Carcinoma
The chromosomal abnormalities that are most commonly identified in bladder cancer have been exploited to develop molecular-based screening tests for urothelial cancer. The most common of these is a fluorescent in situ hybridization based test that can be done on urine (UroVysion”). Aneuploidy of chromosomes 3,7,17 and loss of the p16 locus on 9p12 are evaluated. The test is a helpful adjuvant to urine cytology and clinical follow-up of patients with signs and symptoms or a history of bladder cancer.
The most common types of bladder tumors are urothelial (transitional cell) neoplasms. These show a variety of growth patterns and degrees of cytologic atypia. There are basically three different presentations of bladder neoplasms.
Low-grade noninvasive papillary lesions (papilloma, low-grade carcinoma) usually present with blood in the urine (hematuria). Cystoscopy discloses single or multiple lesions having an exophytic, delicate papillary pattern of growth, which do not invade into the bladder wall. Microscopically, these tumors show increased number of layers of urothelial cells in a papillary architecture, and have only mild cytologic atypia. These are usually treated by simple excision. These lesions may recur but only rarely progress to high-grade invasive tumors. Patients undergo surveillance cystoscopy to look for recurrent tumors.
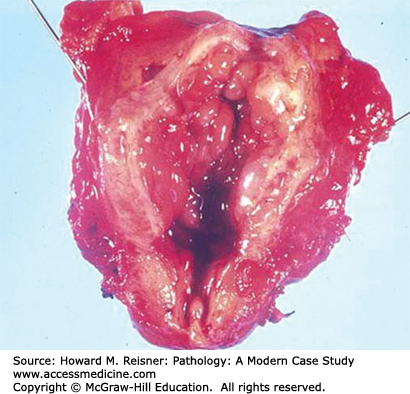
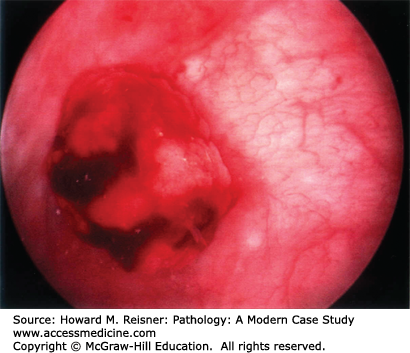
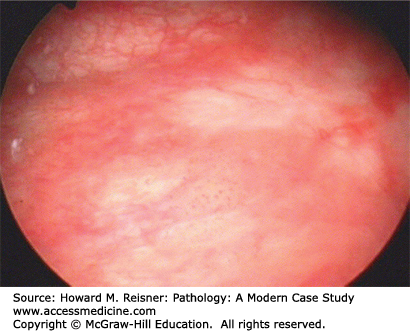
Urothelial carcinoma in situ is a flat lesion that contains malignant cells confined by the basement membrane of the epithelium without invasion, and does not have a papillary architecture. These are grossly seen as red patches or diffuse erythema of the bladder and mimic the gross appearance of cystitis. This lesion usually presents with hematuria and painful urination. Malignant cells may be seen in the urine when examined cytologically (Figure 13-6). These lesions often progress to invasive tumors if left untreated. Treatment is usually with topical immunotherapy using attenuated mycobacterium BCG instilled in the bladder or topical chemotherapy. In some cases the lesion regresses, but if it does not the patient usually undergoes cystectomy (see Figures 13-9 and 13-10).
Invasive carcinomas are almost always high-grade tumors, which often have invaded into the muscularis of the bladder at presentation. Patients usually present with hematuria, sometimes also with pelvic pain or obstruction of one or both ureters. Muscle invasive bladder cancer is aggressive and long-term survival is poor. Prognosis of bladder cancer depends on stage and grade. Stage is the most important factor, but within any given stage grade has a significant contribution.
Unusual variants of bladder cancer include squamous cell carcinoma, which is associated with schistosomiasis, and rarely in the setting of chronic urinary tract infections (UTIs) accompanied by squamous metaplasia, such as occurs in paraplegics with indwelling catheters. Adenocarcinomas are associated with glandular metaplasia, such as with exstrophy, or associated with urachal remnants. Sarcomas, which arise from the stroma of the bladder, are very rare in adults, but are relatively more common in children where embryonal rhabdomyosarcoma is the most frequent example. Occasionally, other pelvic tumors such as cervical or rectal cancer can involve the bladder by direct extension, or the bladder can be involved with metastatic tumors.
RENAL PELVIS AND URETER
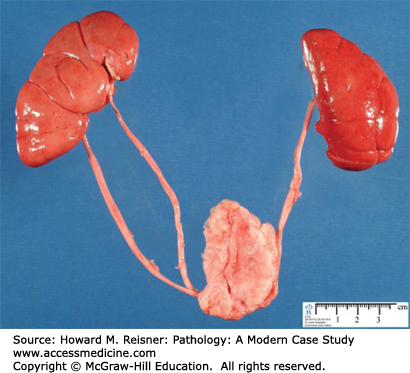
Duplication of the ureter is due to multiple ureteric buds, which occur in approximately 1% of the general population, but in up to 10% of children who are investigated for UTIs. Duplication may be complete (two entirely separate ureters on one side each entering the bladder independently see Figure 13-11) or incomplete (one ureter entering the bladder, branching before the collecting system). Complete duplication is more likely to be symptomatic, with obstruction of the upper pole ureter and incompetence (urine reflux) of the lower pole ureter the common presentation.
CASE 13-3Duplication of the Ureter
A 2-year-old boy is referred to a pediatric urologist for multiple episodes of UTI. Abdominal ultrasound shows dilatation of the collecting system of the upper pole of the kidney with no dilation of the lower pole. Voiding cystoureterography shows reflex into the lower pole of the kidney. This suggests the possibility of duplication of the ureter with obstruction of the upper pole ureter and reflex of the lower pole ureters (Figure 13-11).
Congenital Obstruction, particularly of the ureteropelvic junction, is a common cause of hydronephrosis in children. Abnormal amounts of smooth muscle or fibrous tissue replace the muscle of the ureteropelvic junction in such cases. Other causes of congenital obstruction are ureteric valves (transverse folds across the lumen of the ureters) or congenital atresia (stenosis of the ureter). Agenesis of the ureter results in agenesis of the corresponding kidney, since the kidney is formed by induction of the mesonephric blastema by the ureteric bud. Congenital dilatation of the ureter, known as congenital megaureter, is due to an adynamic segment of ureter that fails to contract in a coordinated fashion to aid the flow of urine.
Obstruction of the ureter may intrinsic (within the lumen of the ureter) or extrinsic (outside the ureter). Intrinsic causes include tumors, stones, blood clots and inflammation, and fibrosis. Extrinsic causes include anything that partially or completely compresses the ureter, such as extraureteral tumors, fibrosis (especially idiopathic retroperitoneal fibrosis), endometriosis, and occasionally pregnancy.
The most common tumor of the renal pelvis and ureter is urothelial carcinoma (transitional cell carcinoma). These are biologically similar to bladder urothelial carcinoma, and discussed in more detail in the bladder section above. Involvement of the ureter or renal pelvis is much less common than the bladder.
PRINCIPAL DISEASES OF THE TESTIS
QUICK REVIEW Normal Anatomy and Histology
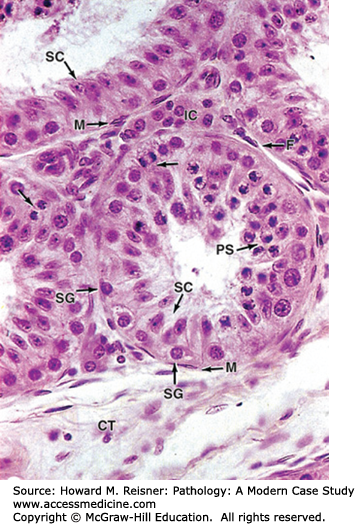
The testes begin intra-abdominally, but descend to the scrotum usually in the late intrauterine period. The scrotal location allows the testes to function at a lower temperature, which is necessary for normal sperm development. The testes are composed of coiled seminiferous tubules present in a loose stroma that contains Leydig cells. Within the seminiferous tubules sperm are produced from germ cells by meiosis. Germ cells progress from spermatogonia to primary spermatocytes to secondary spermatocytes to spermatids to spermatozoa, depending on the stage of meiosis. Also within the seminiferous tubules are Sertoli cells, which nourish sperm and secrete inhibin that helps regulate pituitary gonadotropins (FSH and LH). The stromal Leydig cells secrete testosterone, the male androgenic hormone. The testis is covered by a tough white fibrous capsule, the tunica albuginea. Adjacent to the testis is the epididymis, which is a storage sac for sperm. The outflow of sperm is through the vas deferens in the spermatic cord to the urethra (Figure 13-12).
FIGURE 13-12
Photomicrograph of normal testis histology (detail of seminiferous tubule and interstitial cells). The micrograph shows seminiferous tubules surrounded by connective tissue (CT), containing many large rounded or polygonal interstitial cells (Leydig cells) (IC) secreting androgens. Immediately surrounding each tubule are flattened myoid cells (M), which contract to help move sperm out of the tubule, and layers of fibroblasts (F). Inside the tubule itself is a unique seminiferous epithelium composed of columnar supporting cells called Sertoli cells (SC), which usually have oval nuclei and distinct nucleoli, and germ cells of the spermatogenic lineage. Prominent among the latter are spermatogonia (SG), diploid cells always located near the basement membrane, and primary spermatocytes (PS) that are undergoing meiosis closer to the lumen of the tubule. At the upper left corner is a portion of a straight tubule, which lacks germ cells and consists solely of Sertoli cells. (Mescher et al. Junqueira’s Basic Histology Text & Atlas, 12th edition. New York, NY: McGraw Hill Lange. 2008;374)
Male infertility is a complex topic, and only the high points will be touched on here. Male infertility can be divided into pretesticular causes (such as endocrine and metabolic causes), testicular causes (intrinsic to the testis), and post-testicular causes (blockages of the excretory ducts).
Pretesticular causes include disorders of the hypothalamus, pituitary, and peripheral organs that interfere with the hormone cascade necessary for normal testicular functioning. Common examples are (1) tumors involving the pituitary or impinging on the hypothalamus, (2) a systemic disease or medication that suppresses the release of hormones or has antiandrogenic effects, and (3) idiopathic failure of production of gonadotrophic releasing hormone by the hypothalamus (idiopathic hypogonadotropic hypogonadism).
CASE 13-4 Germ Cell Aplasia
A 32-year-old married male visits a urologist because he and his wife have not been able to conceive after 3 years of trying. The patient’s wife had been previously pregnant with a different partner. Physical examination shows no abnormalities, and past medical history and social history are unrevealing. Semen analysis shows no sperm present. Serum LH, FSH, testosterone, and prolactin are all normal. Transrectal and scrotal ultrasound show no abnormalities, specifically normal sized testis were seen with no evidence of varicocele or ejaculatory duct obstruction. Vasography shows patent excretory ducts bilaterally. Testicular biopsy is performed to further evaluate the testis and potentially retrieve sperm for in vitro fertilization. Biopsy shows normal sized seminiferous tubules with no germ cells and only Sertoli cells (Figure 13-13).
FIGURE 13-13
Photomicrograph of testis with germ cell aplasia. (A) Note complete absence of spermatogonia around periphery of the seminiferous tubules. Refer to Figure 13-12 (normal) for comparison of Histologic details.
Testicular causes of infertility are multifactorial and are both congenital and noncongenital. Cryptorchidism, the most common pediatric genital-associated defect, is associated with sterility when left untreated. Causes of cryptorchidism (see below) are generally multifactorial lacking well-defined genetic etiology. Infertility associated with chromosomal abnormalities includes Klinefelter syndrome [XXY], Y chromosome microdeletions, which may result in germ cell aplasia (Sertoli cell only syndrome see Figure 13-13), Down syndrome, and a number of single gene mutations. Examples of the latter include myotonic dystrophy, which may be associated with testicular atrophy and Noonan Syndrome, most often caused by an autosomal dominant condition resulting in cryptorchidism. Noncongenital causes of infertility include varicocele, trauma, chemotherapy, radiation, and inflammation of the testis (orchitis).
Post-testicular causes include problems with sperm transport through the excretory ducts of the testis, and include congenital and acquired blockages of the ducts, cystic fibrosis, and immotile cilia such as is seen with Kartagener syndrome.
Cryptorchidism is failure of one or both testes to descend into the scrotum. It is more common in premature than full-term infants. In many of these children, the testis will descend spontaneously in the first year of life. If it does not, the testis is usually placed in the correct position surgically (orchiopexy). Uncorrected cryptorchid testes are smaller than normal, contain smaller seminiferous tubules and fewer germ cells, and show a thickened basement membrane and increased stromal fibrosis (Figure 13-14). Patients with one cryptorchid testis usually have decreased sperm counts, and often are infertile if cryptorchidism is bilateral. The principal consequences of cryptorchidism are infertility and increased risk of developing a germ cell neoplasm. Orchiopexy may not significantly reduce this risk.
FIGURE 13-14
Photomicrograph of cryptorchidism. Seminiferous tubules are smaller than normal and contain few (if any) germ cells. Leydig cells are increased in number and hypertrophic having a vacuolated cytoplasm (arrow). There is increased stromal fibrosis and a thickened basement membranes of the tubules (line). Seminiferous tubules contain mostly immature Leydig cells having extensive cytoplasmic processes that fill the tubule (*).
Orchitis is inflammation of the testis. It may be associated with epididymitis, occur secondary to a urethral or bladder infection, or may be due to hematogenous spread of organisms. Bacterial orchitis is usually associated with epididymitis secondary to gram-negative bacteria. Syphilis, tuberculosis, and mumps are other infectious diseases that involve the testis, usually hematogenously.
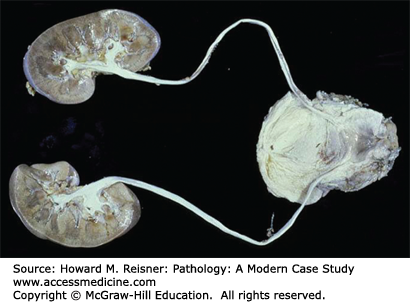
Torsion of the testis is twisting of the spermatic cord within the scrotum, causing vascular compression of the vessels contained in the spermatic cord, which eventuates in hemorrhagic infarction of the testis (Figures 13-15 and 13-16). This is an exquisitely painful condition and a medical emergency. Torsion is most often seen in young men engaging in vigorous exercise. Surgery to untwist the spermatic cord and “tack down” the testis in the scrotum is undertaken if the condition is caught before the testis infarcts, otherwise the necrotic testis is removed.
The most common tumors of the testis are germ cell tumors. Unlike most solid organ tumors, testicular germ cell tumors usually present in young adult life (especially age 25–45). Pathogenesis of testicular carcinoma is unknown in most cases. However, men who have or have had cryptorchidism or genetic syndromes that cause gonadal dysgenesis are at increased risk. The precursor lesion of testicular germ cell tumors is intratubular germ cell neoplasia, which consists of malignant germ cells within the seminiferous tubules without invasion of the stroma. Occasionally, this in situ lesion is found when the testis is biopsied for infertility.
CASE 13-5 Mixed Nonseminomatous Germ Cell Tumor of the Testis
A 23-year-old paratrooper presents to his medical officer with a unilateral 4 cm painless testicular mass. Ultrasound shows that the mass is solid and intratesticular. Serum tumor markers are drawn, and show that both the serum beta-human choriogonadotropin (b-HCG) and alpha-fetoprotein (AFP) are elevated (541 units and 370 units, respectively, with normal being less than 10 units and less than 5 units, respectively). The patient is taken to surgery and a radical orchiectomy is performed, which consists of an incision made into the inguinal area; the testis drawn up into the incision by the spermatic cord, the testis inspected, the spermatic cord clamped, and cut; and the testis with attached epididymis and spermatic cord removed and sent to pathology. Grossly, an intratesticular nodule is present, and microscopically a mixed germ cell tumor is seen, with components of embryonal carcinoma, yolk sac tumor, choriocarcinoma, and teratoma (Figures 13-17 and 13-18). After surgery the tumor markers remained elevated, and CT scan of the abdomen shows retroperitoneal lymphadenopathy. Three cycles of chemotherapy are given and the lymphadenopathy regresses and the tumor markers return to normal baseline.
FIGURE 13-17
Gross photo of nonseminomatous germ cell tumor: Heterogeneous tumor components occupy almost all of this “bivalved” (transected and spread open) testis. The blue color (upper margin of specimen) represents “inking” (ink placed on the external margin) by the surgical pathologist.