Unusual Clinical Presentation of Carcinoma
SYED A. HODA
Breast carcinoma can have myriad clinical presentations. In this chapter, the salient clinical and pathologic aspects of breast carcinomas that present during pregnancy or lactation, in patients at relative extremes of ages, within fibroepithelial tumors, in ectopic breast tissue, with an “inflammatory” appearance, with axillary nodal involvement, or in transverse rectus abdominus muscle (TRAM) flaps, are presented.
CARCINOMA IN PREGNANCY AND LACTATION
Breast carcinoma is the most common malignant neoplasm encountered during pregnancy, afflicting one in 3,000 pregnancies.1 The frequency of coincident pregnancy in women with breast carcinoma is 1% to 3%.2 The average age of women who have breast cancer in pregnancy is in the mid- to late 30s.2 About 6% of women with breast carcinoma diagnosed by age 35 are pregnant when the tumor is detected.3 With the trend for women in some sociocultural groups to delay pregnancy to their late 30s and 40s, it is likely that coincident pregnancy and breast carcinoma will be increasingly encountered.1 Breast carcinoma has been reported in pregnant teenage girls.4,5 Data from one study suggest that women from BRCA1-positive families may be at increased risk of developing breast carcinoma during and after pregnancy.6
Clinical Presentation
The usual presenting symptom is a painless mass that may be obscured by pregnancy-associated physiologic changes in the breast. This factor can contribute to delay in seeking medical attention on the part of the patient, as well as physician delay in recognizing the presence of a neoplasm and obtaining a biopsy. In one study, more than 50% of patients with breast carcinoma diagnosed postpartum had a palpable mass detected and followed during pregnancy.7
With appropriate precautions, mammography can be performed during pregnancy.8,9 The effectiveness of mammography may be reduced during pregnancy because of increased parenchymal density.10 Liberman et al.11 reported that the sensitivity of mammography for detecting carcinoma during pregnancy or within 1 year postpartum was 78%. In one study, ultrasound was 100% sensitive as a method for detecting solid tumors in a series of pregnant women, and is now regarded as the preferred initial diagnostic procedure in this setting.12,13 Ultrasound is also essential for monitoring response to neoadjuvant chemotherapy during pregnancy and for evaluating axillary lymph nodes (ALNs).14 Invasive carcinomas can be identified in lactating breast tissue by magnetic resonance imaging (MRI),15 but uncertainty about the effects of gadolinium on the fetus and issues related to positioning the pregnant patient have raised concern about this diagnostic procedure.16
Pathology
A diagnosis of carcinoma during pregnancy and lactation can be made by fine-needle aspiration (FNA) biopsy.17 However, caution should be exercised when interpreting cytology specimens in this setting, because physiologically altered non-neoplastic mammary epithelial cells can appear atypical in cytologic preparations in this setting, and the material often consists of abundant dyscohesive cells.18 For these reasons, needle core biopsy or excisional biopsy are preferable for definitive diagnosis.
The histologic spectrum of pregnancy-associated breast carcinoma is not significantly different from breast carcinoma unrelated to pregnancy in women of a comparable age.7,16,19,20,21 Invasive ductal carcinoma (IDC) is present in about 90% of both groups. There were small numbers of patients with invasive lobular, mucinous, medullary, and other types of carcinoma. Tumors are significantly larger, and vascular invasion as well as axillary nodal involvement is more frequent in the pregnancy group. In one case-control study, intraductal carcinoma was present in 4.8% of control patients and in 1.6% of women with pregnancy-related carcinoma.19 ALN metastases are present in 60% to 70% of women with pregnancy-related breast carcinoma.7,19,22
Prognostic Markers
Estrogen receptors (ER) and progesterone receptors (PR) are significantly more often negative in carcinomas from pregnant and lactating women than in tumors from nonpregnant age-matched controls.16,19,22,23,24,25,26 A substantial proportion of such carcinomas, ranging from 44% to 58%, are HER2-positive.24,25,26
Treatment and Prognosis
Although the primary treatment has generally been surgical, the use of adjuvant chemotherapy and breast conservation is an increasingly exercised option, depending on the circumstances in a particular case.16 Surgery and chemotherapy are relatively safe treatment options after the fetal organogenesis period of the first 16 weeks has elapsed. Therapeutic irradiation ought to be delayed until after completion of pregnancy.27 However, the use of chemotherapy at any time during pregnancy has been linked to underdevelopment of placenta.28 The most significant obstetrical outcome in women who have received chemotherapy during pregnancy is low birth weight.29 Although no long-term complications have been reported in children whose mothers received chemotherapy for hematologic neoplastic diseases during pregnancy, the effects of fetal in utero exposure to maternal chemotherapy for breast carcinoma have not been well studied.30
In the past, a modified radical mastectomy was performed in most cases for local control, in part to avoid radiation of the fetus during breast conservation therapy.16,31,32,33,34 Radiation should be delayed until after pregnancy.16 Results in 9 patients treated by breast conservation in pregnancy were reported by Kuerer et al.35 The patients were all stage I and stage II, with a median fetal gestation of 7 months. After a median follow-up of 24 months, there were no recurrences in the breast, although three women had distant recurrences.
Thus far, no adverse effects have been reported with the use of either lymphoscintigraphy or methylene blue in pregnancy for the detection of sentinel lymph nodes (SLNs),36,37,38,39,40,41 although the use of lymphoscintigraphy alone has been recommended in this setting.16 In general, breast carcinoma in pregnancy can now be safely and effectively treated; however, management needs to be guided by duration of pregnancy and stage of breast cancer.32
The overall prognosis of women with breast carcinoma diagnosed in pregnancy and lactation is relatively poor owing to the high proportion of patients with nodal metastases.31,42 In one study, axillary nodal metastases were present in 74% of patients younger than 40 years of age with breast carcinoma diagnosed during pregnancy, whereas 37% of nonpregnant patients in the same age group had positive nodes.43 When stratified by stage, some investigators reported no significant difference in outcomes between pregnancy-related and non-pregnancy-related patients of comparable age.7,20,22,44 In a number of reports, 75% to 80% of node-negative patients remained alive or recurrence free with follow-up of 5 to 10 years. In one case-control study, node-negative women in the pregnancy and lactation group had a poorer survival (85%) at 10 years than women with non-pregnancy-associated breast carcinoma (93%), but the outcomes for both groups were favorable.19 The same series reported a greater discrepancy in node-positive cases, with survival of 62% and 37% in the nonpregnant and pregnant groups, respectively. Others also found the prognosis of pregnancy-associated breast carcinoma to be relatively unfavorable after adjustment for tumor size and nodal status.45
The impact of subsequent pregnancy on prognosis in women previously treated for breast carcinoma remains uncertain.46 Most studies of this subject conducted retrospectively appear to indicate that the prognosis for such patients is the same as or better than for patients who do not become pregnant.47,48 Women who have received chemotherapy are generally advised to delay pregnancy for at least 6 months before attempting to conceive.49 One case-control study compared 53 women who became pregnant after treatment of breast carcinoma with a cohort without subsequent pregnancy, matched for stage of disease at diagnosis and a disease-free survival (DFS) at least as long as the interval to pregnancy in the study individual.50 There were 5 deaths due to breast carcinoma among 53 women (9.6%) with subsequent pregnancies and 34 deaths among 265 controls (13%). The relative risk (RR) of death due to breast carcinoma in the subsequent pregnancy group was 0.8 (95% confidence interval [CI], 0.3 to 2.3), a result indicative of no increase in risk associated with subsequent pregnancy. A prospective study will be required to fully evaluate this issue, especially in the context of current management practices.
An unusual complication of pregnancy concurrent with or subsequent to the diagnosis of breast carcinoma is the development of placental metastases. This is most likely to occur in women who have disseminated metastatic tumors.51,52,53 Gross evidence of metastatic carcinoma is usually apparent on the placental surface, and microscopic examination discloses tumor cells in the intervillous spaces, rarely with villous invasion (Fig. 33.1).
BREAST CARCINOMA IN “YOUNGER” AND “OLDER” WOMEN
The average age at diagnosis of patients with breast carcinoma is in the mid-50s. The ages of the majority of affected women are within two decades above or below this midpoint. Within this framework, the extremes of age may be considered younger than 35 years and older than 75 years.
Breast carcinoma is widely thought to have a relatively poor prognosis in women younger than 35 years of age, whereas in those older than 75 it has been described as an indolent disease. Many published studies of this issue are not easily compared because of differences in defining age extremes or in the treatment that patients received. These are important considerations, especially when comparing data from the era when therapy consisted of surgery alone with recent data including neoadjuvant and adjuvant therapy, breast conservation, and radiation therapy. Data obtained
from a statewide tumor registry for patients treated between 1985 and 1992 suggest that age-related differences in prognosis are influenced by the stage at diagnosis.54
from a statewide tumor registry for patients treated between 1985 and 1992 suggest that age-related differences in prognosis are influenced by the stage at diagnosis.54
Patients Younger Than 40 Years
Simmons et al.55 investigated the incidence of breast carcinoma in women younger than 25 years of age by reviewing data from a Minnesota county between 1935 and 2005 for histologically confirmed cases. The four cases diagnosed over the 1,201,539 person-years yielded an annual ageadjusted incidence of 3.2 per million (95% CI, 0.1 to 6.2). Since all patients were in the 20- to 24-year age group, the age-specific incidence in this subset was 16.2 per million. The authors noted that delay in diagnosis was a common feature in these cases.
Kothari et al.56 reported on 15 women 25 years or younger at the time of diagnosis. Two patients had intraductal carcinoma. None of the invasive carcinomas in 13 patients were low grade. Nine (69%) of the 13 women with invasive carcinoma died as a result of recurrent carcinoma—with a median DFS of 86 months. There was no statistically significant difference in overall survival (OS) between women 25 years or younger and those 26 to 35 years of age at the time of diagnosis.
Feldman and Welch57 studied 29 women who were younger than 30 years when diagnosed and treated for breast carcinoma. The patients were identified between 1953 and 1983 in the records of an urban teaching hospital. Age at diagnosis ranged from 20 to 29 years. Seven patients (26%) were pregnant at the time of diagnosis. Delay in diagnosis was probably a factor in the prognosis of these patients since the stage at diagnosis was II or higher in 26 of 27 cases with documented data. Twenty-two patients (76%) died of breast carcinoma, including three who developed recurrences 12.7, 14.6, and 19.9 years after diagnosis. It should be noted that virtually all patients were treated by mastectomy and none received systemic adjuvant therapy.
A larger series of patients 30 years or younger at diagnosis, consisting of 185 women, was reported by Xiong et al.58 The distribution of patients by stage was as follows: stage I, 11%; stage II, 45%; stage III, 38%; and stage IV, 6%. Treatment consisted of various combinations of mastectomy or breast-conserving surgery with adjuvant or neoadjuvant chemotherapy and radiotherapy. The 5-year OS rates by stage were stage I, 87%; stage II, 60%; stage III, 42%; and stage IV, 16%. When compared with control patients identified in the National Cancer Data Base, women diagnosed at age 30 or younger had poorer 5-year OS rates.
A retrospective case-controlled study by Peng et al.59 concluded that diagnosis at or before 35 years of age was an independent negative prognostic risk factor. They compared 551 women 35 years or younger who had operable breast carcinoma to a cohort of women 36 to 50 years of age at diagnosis matched for year of diagnosis, family history, pathologic stage at diagnosis, hormone receptor status and adjuvant therapy. The younger women had a significantly shorter disease-free interval to first recurrence (median 23.2 vs. 28.4 months), a lower DFS (63.7% vs. 74.7%), and lesser OS (79.5% vs. 85.6%).
Liukkonen et al.60 studied 212 Finnish women treated between 1997 and 2007 for breast carcinoma who were younger than 35 years. At diagnosis, 117 (55%) had axillary nodal metastases and 14 (7%) had distant metastases. One hundred and forty (65%) women were treated with mastectomy and 68 with breast conservation surgery. Postoperative treatment included chemotherapy, endocrine therapy, and radiation, singly or in combination. Local recurrence occurred in 10 (15%) of women treated with breast conservation surgery, and 8 (6%) of patients treated
with mastectomy. The disease-free interval was shorter in patients with hormone receptor-positive carcinoma after a median follow-up of 78 months. The overall 5-year survival was 80%, suggesting that the prognosis of patients in this age group is improved by earlier diagnosis and the use of modern treatment modalities in addition to surgery.
with mastectomy. The disease-free interval was shorter in patients with hormone receptor-positive carcinoma after a median follow-up of 78 months. The overall 5-year survival was 80%, suggesting that the prognosis of patients in this age group is improved by earlier diagnosis and the use of modern treatment modalities in addition to surgery.
Patients 40 to 49 Years of Age
Most studies of clinical issues in the diagnosis of breast carcinoma in younger women have focused on the relatively large group of patients 40 to 49 years of age. A report of 809 consecutive patients biopsied for nonpalpable, mammographically detected lesions revealed carcinoma in 5% of biopsies prior to age 40, in 15% of biopsies in the 40- to 49-year age group, and in 34% of biopsies from women older than 50 years.61 Twenty-five percent of carcinomas in women 40 to 49 years old and 16% in women 50 years or older were noninvasive. Mean tumor size was the same in both groups (1.5 cm), but nodal metastases were present more often in the 40- to 49-year age group (25%) than in the group 50 years or older (17%).
McPherson et al.62 investigated the relationship of method of tumor detection to prognosis in women 40 to 49 years of age using a database of patients diagnosed in North Dakota, South Dakota, and Minnesota. When compared with the risk of dying from carcinomas detected by mammography, the RRs of dying from carcinomas detected by breast selfexamination (BSE) (2.5), clinical breast exam (CE) (2.7), or discovered by the patient incidentally (2.8) were significantly greater. The mean size of mammographically detected tumors (1.9 cm) was significantly smaller than those in the CE (2.3 cm), BSE (2.8 cm), and incidental (2.9 cm) groups. After adjusting for stage (tumor size and nodal status), the RRs of dying of carcinomas were greater when detected by BSE (1.5), CE (1.9), or incidentally (1.6), when compared with tumors detected by mammography. These results suggest that mammography makes a contribution to improving the prognosis of women with carcinoma 40 to 49 years of age. The implications of these observations for mammographic screening in this age group and in women younger than 40 years remain controversial.
Clinical problems encountered in the diagnosis of breast carcinoma in women 49 years and younger were detailed in a report by Lannin et al.63 The authors analyzed the results of mammography and physical examination in a consecutive series of patients evaluated in a university hospital clinic in order to compare women 20 to 49 years of age with those 50 years or older. The positive predictive value (PPV) of mammography was 28% for women younger than 50 and 53% in those 50 years or older. The PPV of an abnormal physical examination resulting in biopsy was 11% and 57% in women younger than 50 years and 50 or older, respectively. There was also a statistically significant difference in the sensitivity of mammography between patients younger than and 50 years or older (68% and 91%, respectively). The sensitivity of physical examination did not differ significantly between the two groups. This discrepancy was not observed between nonpalpable and palpable tumors in women younger than 50 years (mean tumor size, 4.0 and 3.4 cm, respectively). These results led the authors to conclude that physical examination and mammography were less sensitive in women 20 to 49 years old when compared with women 50 years or older. They suggested that “tumors in young women are nonpalpable, not because they are small, but because of background density of the mammary tissue or because of the more diffuse growth pattern of tumors at this age. These are exactly the same reasons mammography is less sensitive in young women.” The addition of FNA or needle core biopsy for abnormalities detected by mammography and clinical examination constitutes the “triple test” for the diagnosis of breast tumors, a method that improves diagnostic accuracy, especially in younger women.64
Pathology
Most pathologic features of breast carcinoma do not differ appreciably in adults who are relatively young or old.65,66,67,68 Tumor size is not significantly different when young and elderly patients are compared.67 Approximately 50% of patients have tumors 2 cm or smaller, 40% have tumors in the 2.1- to 5.0-cm range, and the rest have tumors larger than 5 cm. The left breast is more often affected than the right in both age extremes. The location of the tumor (lateral vs. medial-central), the overall frequency of bilaterality, and concurrent bilaterality are not significantly different at the extremes of the age distribution.
Several differences with respect to tumor type exist at the extremes of age.66 Patients younger than 35 have a higher proportion of medullary carcinoma, and lower proportions of infiltrating lobular (2.0% vs. 11.0%) and of mucinous carcinoma (1.0% vs. 7.0%), in comparison with patients older than 75. A marked lymphocytic reaction occurs in a higher proportion of women younger than 35 than in the elderly group (34% vs. 12%).
Collins et al.69 analyzed clinical and pathologic data for 657 patients with intraductal carcinoma (ductal carcinoma in situ [DCIS]) to identify features that might explain the greater risk for local recurrence in young women after breastconserving therapy. Four age groups were compared, with the youngest consisting of 111 women less than 45 years of age at diagnosis, who proved to have significantly more extensive DCIS and more frequent lobular cancerization than women older than 45. DCIS was detected by mammography significantly less often in women younger than 45 years than in any of the older cohorts. There was no statistically significant relationship between age and the following features of DCIS: architectural type, nuclear grade, comedonecrosis, or the expression of receptors for ER, PR, or epidermal growth factor receptor 2 (EGFR2).
Prognostic and Predictive Markers in Invasive Carcinoma
Studies of growth rate and tumor cell kinetics suggest an inverse relationship between patient age at diagnosis and the proliferative activity in the invasive carcinoma.70,71 Growth rate tends to
be reduced in breast carcinomas that arise in elderly women.71 Others have reported that the presence of ALN metastases in breast carcinoma patients 34 years or younger is significantly related to p53 positivity and high proliferative index.72
be reduced in breast carcinomas that arise in elderly women.71 Others have reported that the presence of ALN metastases in breast carcinoma patients 34 years or younger is significantly related to p53 positivity and high proliferative index.72
Walker et al.73 found an inverse relationship between p53 immunopositivity and age, with positive staining in 67% and 37% of tumors from women 25 to 29 and 50 to 67 years of age, respectively. Proliferative rate, assessed by Ki67 immunostaining, was also inversely related to age, with 72% of tumors in patients 25 to 29 classified as “high” compared with 40% in the group 50 to 67 years of age.
The proportion of ER-positive invasive carcinomas is higher in postmenopausal than in premenopausal women, and there is evidence indicating that the growth rate and positive ER status of breast carcinomas are inversely related.71 Although breast carcinomas in younger women are now more often detected before involving lymph nodes than 10 years ago, a larger percentage is triple-negative.74 The proportion of ER– and PR-positive tumors does not increase significantly with advancing age in postmenopausal women 65 years or older.75 Gennari et al.76 reported that the frequency of estrogen and progesterone positivity was significantly higher in postmenopausal women 65 years of age or older when compared with postmenopausal women 50 to 64 years old. The older postmenopausal women had a significantly lower frequency of HER2-positive tumors. These observations appear to support the perception that breast carcinoma tends to have less aggressive biologic features and a more favorable clinical course in the elderly. Nonetheless, no significant differences in prognosis were observed when patients younger than 35 and older than 75 were matched on the basis of tumor stage.66
Breast Conservation Therapy
Women 40 years of age or younger are more likely than older patients to develop breast recurrences after breastconserving surgery and radiotherapy for invasive carcinoma.77,78,79,80,81 This phenomenon has been attributed to more frequent poorly differentiated carcinomas in this age group, difficulty in determining extent of carcinoma intraoperatively, and a high prevalence of carcinomas with an extensive intraductal component or lymphatic emboli in peritumoral tissue.78 The addition of adjuvant chemotherapy appears to lower the risk of breast recurrence in women younger than 35 years who are treated by breast conservation.79,82,83 Chest wall irradiation has been recommended if carcinoma is present at or close to (less than 5 mm) the deep margin of a mastectomy.84 The risk of breast recurrence after breast conservation does not appear to be affected by a family history of breast cancer.85
Vicini et al.86 reported that patients younger than 45 years of age had a significantly greater risk of breast recurrence after conservation therapy (excision and radiotherapy) for intraductal carcinoma than women who were 45 years or older. The frequency of invasive recurrence was substantially greater in the younger age group. When the volume of tissue was considered in the analysis, age at diagnosis proved not to be significantly related to recurrence risk, and it was concluded that the higher local failure rate in patients younger than 45 was related to smaller excision volumes in this age group.
Arvold et al.87 studied 1,434 consecutive patients with invasive breast cancer who received breast conservation therapy over a 10-year period ending 2006. Ninety-one percent received adjuvant systemic therapy. The median follow-up was 85 months, and the overall 5-year cumulative incidence of local recurrence was 2.1%. The 5-year cumulative incidence of local recurrence was 5.0% for ages 23 to 46 years; 2.2% for ages 47 to 54 years; 0.9% for ages 55 to 63 years; and 0.6% for ages 64 to 88 years. On multivariable analysis, increasing age was associated with decreased risk of local recurrence.
Carcinoma in Elderly Women
The Cancer and Leukemia Group B (CALGB) trial 9343 examined the contribution of radiation after lumpectomy in women aged 70 and older with ER-positive node-negative breast carcinomas that were 2 cm or smaller.88 After tumor excision, the patients were randomized to tamoxifen alone versus radiation plus tamoxifen. An update of this study with a 10.5-year median follow-up showed that 98% of the radiation plus tamoxifen group and 92% of the tamoxifenonly group were recurrence free.89 Based on the 6% lower frequency of ipsilateral breast tumor recurrence for the radiation group, it was estimated that 300 women would have to be radiated to prevent 20 local recurrences. The fact that the two groups did not differ significantly in overall 10-year survival and breast-cancer-specific survival suggests that the small difference in the frequency of breast recurrence did not significantly affect survival 10 years after initial treatment.
Results of the ongoing Postoperative Radiation in Minimal Risk Elderly Patients (PRIME II) trial90 in the United Kingdom may further define the effect of omission of radiation in elderly patients with hormone receptor node-negative invasive carcinomas. Until then, the advantages of radiation after breast conservation therapy in this subset of elderly patients should be weighed against its morbidities, and the decision to radiate should be individualized.
Genetic Considerations
Some special considerations are to be kept in mind regarding genetic abnormalities and breast carcinoma in young adult women.
“Secretory carcinoma” is the most common malignant epithelial neoplasm encountered in children. Its occurrence in younger patients accounts for the previously used term juvenile carcinoma, but it can be found in adult women of all ages. Because secretory carcinoma almost always has a balanced chromosomal translocation, t(12:15)(p13;q25) that leads to fusion of the ETV6 and NTRK3 genes,91 it has been referred to as “a genetically defined carcinoma entity.”92
More information about secretory carcinoma can be found in Chapter 22.
More information about secretory carcinoma can be found in Chapter 22.
Among the 132 BRCA-positive women with breast carcinoma who participated in a high-risk protocol at The University of Texas M.D. Anderson Cancer Center, 106 second-generation women could be paired with a family member in the previous (first) generation who was diagnosed with a BRCA-related carcinoma in either breast or ovarian carcinoma.93 The median age of carcinoma diagnosis in the first-generation patients was 48 years (range, 30 to 72 years) and in the second-generation patients, it was 42 years (range, 28 to 55 years). This trend was found in subgroups with either a BRCA1 or BRCA2 mutation. The statistically significant difference in age at diagnosis suggests that BRCA-mutation-related carcinomas in at least one subsequent generation occur at an earlier age after the first case is identified. The prognosis of breast carcinoma patients in BRCA 1 and BRCA 2 carriers has been controversial. In a large international population-based cohort study BRCA1 and BRCA2 mutation carriers were found to have no significant difference in outcome when compared with patients with sporadic breast carcinoma after adjusting for age, stage and grade of tumor, lymph node and hormone receptor status, and year of diagnosis.94
Li-Fraumeni syndrome is a rare autosomal dominant disorder that is linked to germline mutations of the p53 tumor suppressor gene. The syndrome increases susceptibility to certain forms of cancer, including those of the breast, bone, and soft tissues. A cohort of eight breast carcinoma patients with a median age at diagnosis of 30 from Li-Fraumeni families with the associated germline p53 mutations was studied in France.95 Six of eight received radiation (including three after mastectomy). After a median follow-up of 6 years, an extraordinarily high incidence of subsequent events occurred in the six radiated patients consisting of three ipsilateral breast recurrences; three contralateral breast carcinomas; two radiation-associated sarcomas; one thyroid carcinoma in field. The data suggest that bilateral mastectomy is appropriate and that radiation therapy is contraindicated in this setting because the patients appear to have a genetic predisposition to develop radiationassociated malignant neoplasms.
CARCINOMA ARISING IN FIBROEPITHELIAL NEOPLASMS
Fibroepithelial neoplasms consist of proliferating epithelial and stromal mammary tissues. Fibroadenomas (FA) arise from the stroma and epithelium of lobular-terminal duct units, whereas phyllodes tumors (PT) are composed predominantly of periductal stroma and duct epithelium.
In 1931, Cheatle and Cutler96 described carcinoma arising in a FA, and similar lesions were reported in 1940 by Harrington and Miller.97 The first series, consisting of 26 patients, was published in 1967.98 Numerous cases have been subsequently reported.99,100,101,102,103 Carcinoma occurs in less than 0.5% of FA,101,104 and in 1% to 2% of PT.101,102
Clinical Presentation
The age of patients with carcinoma arising in a FA ranges from 15 to 70 years, with a mean age of 42 to 44 years.101,104 Women with in situ carcinoma have a mean age of 42 to 45 years, and the mean age for patients with invasive carcinoma in a FA is 47 to 52 years.99 Because patients who have carcinoma in a FA tend to be somewhat older than those with FA that lack carcinoma, the possibility of encountering carcinoma should be anticipated when a FA is excised from a patient 35 years or older. The age distribution of women with carcinoma in or associated with a PT is not appreciably different from women with PT generally, reflecting the older age distribution of PT.
There are no specific clinical or radiologic clues to indicate the presence of in situ carcinoma within a FA or a PT. Invasive carcinoma in or associated with a FA may distort or blur the margin of the tumor in a mammogram.105,106 Rarely, the pattern of calcifications in a FA can suggest intraductal carcinoma.105,107 Carcinoma is more likely to be detected in a fine-needle aspirate from a FA if the carcinomatous component is extensive. The diagnosis depends upon recognizing neoplastic cells in the customary background of benign epithelium and stromal cells obtained from a typical FA.108,109 If the lesion consists of in situ carcinoma limited to a small part of the tumor, there may not be sufficient material in a FNA or a needle core biopsy specimen to be diagnostic.110 Because there are no good clinical indicators of the presence of carcinoma in a fibroepithelial tumor, the diagnosis is generally not suspected until a needle core biopsy has been obtained or the excised tumor has been examined pathologically.
Gross Pathology
When in situ carcinoma is present in a FA or PT, it will often not be apparent on gross inspection.99 FA that harbor carcinoma may not be especially large and many do not exceed 2 cm. Unusual firmness may develop at the site of intraductal carcinoma, particularly in those which are of high-grade with necrosis and calcifications. Invasive carcinoma confined to a FA is generally inconspicuous grossly, but invasion into the adjacent breast tissue can distort the tumor enough to be evident.
Microscopic Pathology
The distinction between atypical hyperplasia and in situ carcinoma in a FA or PT is based on the same criteria that are used to assess epithelial proliferation in the mammary parenchyma. The characteristics of epithelial abnormalities within a FA or PT do not necessarily reflect the proliferative status of the surrounding breast tissue.
Fibroadenoma
The morphology of carcinomas that arise in FA is not peculiar to this setting, but the relative frequency of the types of carcinoma differs from that of carcinomas found in non-fibroepithelial breast parenchyma. In published reports, more than 50% of the affected FA had lobular carcinoma in situ (LCIS)99,100 (Fig. 33.2). Among patients who were treated by mastectomy, LCIS was found in the surrounding
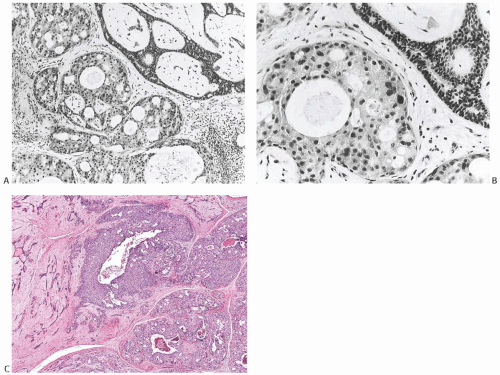
breast tissue in about half of the cases. Nearly 20% had DCIS (Figs. 33.3 and 33.4). IDC accounted for 20% of the cases (Fig. 33.5), and about 10% had invasive lobular carcinoma (ILC) (Fig. 33.6). The IDCs have well-differentiated to moderately differentiated lesions. It is exceedingly unusual for special types of duct carcinoma to arise in a FA or PT. Atypical epithelial lesions in fibroepithelial tumors are prone to having a conspicuous myoepithelial component, and are associated with a variety of findings, including sclerosing adenosis (SA), cysts, apocrine metaplasia, and calcifications, which constitute the so-called complex FA. Petersson et al.111 described a complex FA that gave rise to a low-grade in situ and invasive ductal carcinoma (IDC) associated with columnar cell change.
breast tissue in about half of the cases. Nearly 20% had DCIS (Figs. 33.3 and 33.4). IDC accounted for 20% of the cases (Fig. 33.5), and about 10% had invasive lobular carcinoma (ILC) (Fig. 33.6). The IDCs have well-differentiated to moderately differentiated lesions. It is exceedingly unusual for special types of duct carcinoma to arise in a FA or PT. Atypical epithelial lesions in fibroepithelial tumors are prone to having a conspicuous myoepithelial component, and are associated with a variety of findings, including sclerosing adenosis (SA), cysts, apocrine metaplasia, and calcifications, which constitute the so-called complex FA. Petersson et al.111 described a complex FA that gave rise to a low-grade in situ and invasive ductal carcinoma (IDC) associated with columnar cell change.
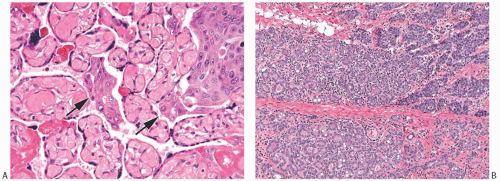
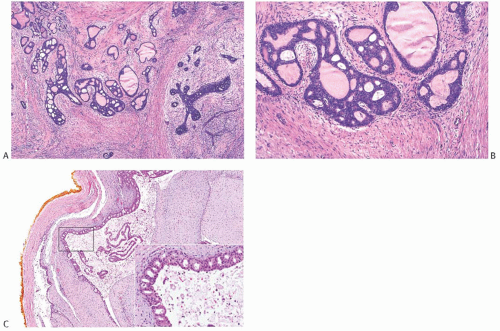
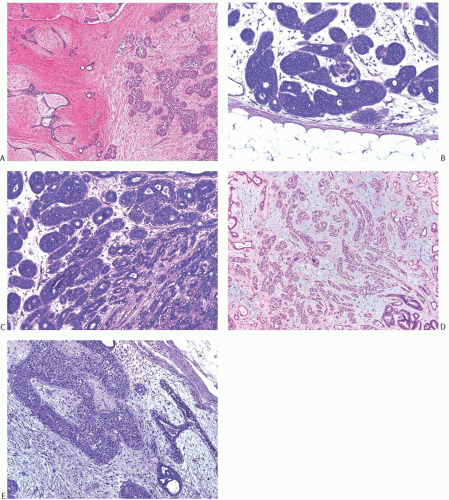 FIG. 33.2. Fibroadenoma, lobular carcinoma in situ. A: The epithelial component of the fibroadenoma is expanded by LCIS (right). B,C: LCIS does not extend beyond the border of this fibroadenoma with edematous stroma and sclerosing adenosis. D: LCIS involving an area of tubular adenosis in another complex fibroadenoma. E: Much of the epithelial element of this fibroadenoma is greatly expanded by LCIS. |
The probability of finding carcinoma in breast tissue outside a FA that is involved by carcinoma has been difficult to determine on the basis of published reports, because many patients were treated only by excisional biopsy. A literature review of 62 published cases found extra-fibroadenomatous carcinoma in 42% of patients.104 Diaz et al.99 reported that the type and amount of carcinoma in a FA and the age at diagnosis were not significant predictors of the likelihood of finding carcinoma in the surrounding breast tissue. Among women treated by mastectomy, carcinoma was limited to the FA in one-third to one-half of cases that had LCIS, DCIS, or ILC.101,104 IDC that arose in a FA involved the surrounding breast tissue in at least 50% of cases. With rare exceptions, the same type of carcinoma has been found in the FA and in the breast tissue. LCIS may be detected in multiple FA in one breast or in bilateral FA.100 ALN metastases have arisen from invasive carcinoma present exclusively within a FA in two cases.97,112 Ten percent to 15% of patients with carcinoma in a FA have had contralateral carcinomas concurrently or previously treated.96,100,101 The opposite breast contained IDC in the majority of these cases. Subsequent contralateral carcinoma has been described in about 6% of cases.99
Phyllodes Tumor
Florid hyperplasia involving epithelial and myoepithelial cells is often encountered in PT. The degree of atypia in the epithelial hyperplasia parallels that of the stromal component in some but not all cases. Mitoses may be seen in hyperplastic epithelial and myoepithelial cells.
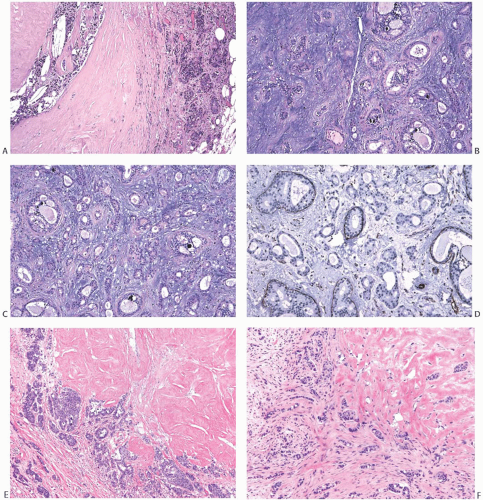
Carcinomas arising in PT are histologically similar to carcinomas developing in FA. LCIS (Fig. 33.7) is less
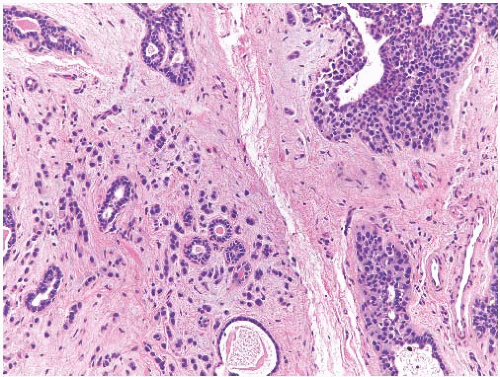
frequent than DCIS (Fig. 33.8). Infiltrating duct carcinomas (Fig. 33.9) have also been described.101,102,113,114,115,116 An IDC arising in a low-grade malignant PT in a 24-year-old woman was the source of isolated tumor cells in an ipsilateral SLN.117 Korula et al.118 described a 51-year-old woman who had DCIS in and around an malignant PT. Lymphatic tumor emboli were found in the PT, and metastatic carcinoma was present in two ALNs, but no invasive tumor was detected in the PT or in the breast. Microinvasive and ILC can also be found in this setting.119 A high-grade malignant PT that contains carcinoma is a form of carcinosarcoma, because these lesions are, by definition, neoplasms that combine carcinomatous and sarcomatous elements derived from the mammary epithelium and stroma. Carcinoma has been found in the surrounding breast tissue concurrently with, or subsequent to, excision of a PT that contained carcinoma.102,120,121
frequent than DCIS (Fig. 33.8). Infiltrating duct carcinomas (Fig. 33.9) have also been described.101,102,113,114,115,116 An IDC arising in a low-grade malignant PT in a 24-year-old woman was the source of isolated tumor cells in an ipsilateral SLN.117 Korula et al.118 described a 51-year-old woman who had DCIS in and around an malignant PT. Lymphatic tumor emboli were found in the PT, and metastatic carcinoma was present in two ALNs, but no invasive tumor was detected in the PT or in the breast. Microinvasive and ILC can also be found in this setting.119 A high-grade malignant PT that contains carcinoma is a form of carcinosarcoma, because these lesions are, by definition, neoplasms that combine carcinomatous and sarcomatous elements derived from the mammary epithelium and stroma. Carcinoma has been found in the surrounding breast tissue concurrently with, or subsequent to, excision of a PT that contained carcinoma.102,120,121
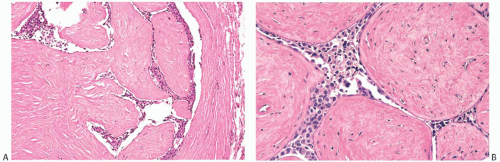 FIG. 33.4. Fibroadenoma, intraductal carcinoma. A,B: The usual epithelium in these two different sclerotic fibroadenomas has been replaced by high-grade DCIS. |
Well-differentiated infiltrating duct carcinoma122 and tubular carcinoma115,123 have been described in PT. The latter case was unusual in that tubular carcinoma was found in the second recurrence of a benign PT. The first recurrence contained LCIS. In the case reported by Quinlan-Davidson,115 LCIS and tubular carcinoma coexisted in a low-grade malignant PT.
Other unusual pathologic presentations have been coexistent DCIS and LCIS in a benign PT,124 invasive carcinoma with ductal, secretory, and squamous components,125 infiltrating duct carcinoma coincidental with but separate from benign PT102 and malignant PT,126 LCIS in a PT with liposarcomatous stroma,127 and microinvasive lobular carcinoma in a benign PT.119 PT that harbor carcinoma are usually benign or low-grade malignant tumors, whereas carcinoma is more often found in breast tissue outside an malignant PT.114,121
There are rare instances of PT with carcinoma that developed after treatment for another malignant neoplasm. Aziz et al.121 described a 43-year-old woman who had carcinoma in a malignant liposarcomatous PT. Approximately 20 years earlier she had received chemotherapy for Hodgkin disease and radiotherapy to the lumbar region. Another woman who developed a liposarcomatous and chondrosarcomatous malignant PT associated with carcinoma when 26 years old had been treated by surgery and chemotherapy without radiotherapy for tibial osteosarcoma 11 years earlier.120
Molecular Analysis
Macher-Goeppinger et al.128 described the results of the molecular analysis of an IDC within an malignant PT. DNA was isolated from the microdissected epithelial and stromal components of the PT, and from the high-grade IDC. Using the multiplex polymerase chain reaction (PCR), comparative allelotyping was performed with a panel of 11 microsatellite markers. Analysis of the data revealed that the stromal component of the PT showed loss of heterozygosity (LOH) at chromosome 16q23, 17q12, 17q25, and 22q13 and that the epithelial element of the tumor shared the loss of 16q23. The invasive carcinoma had lost divergent alleles at 16q23, 17q12, and 17q25. These findings were interpreted as demonstrating a lack of clonality between the malignant PT and the invasive carcinoma that arose within it.
Treatment and Prognosis
There have been very few deaths due to carcinoma arising in a FA, and these have been attributable to IDCs.101,104 Recurrence in the breast following excisional biopsy of a FA that harbored in situ lobular or intraductal carcinoma has been uncommon and appears to be less frequent than when the same lesions that occur outside of FA have been treated only by excisional surgery.99 There are virtually no published data on breast conservation therapy that employed radiation in addition to excisional surgery to treat intraductal carcinoma in a FA. The low frequency of subsequent carcinomas may reflect to some extent the relatively short follow-up, averaging less than 10 years, in most series of patients with carcinoma arising in a FA.
There are no systematic data on the treatment and prognosis of women who had carcinoma arising in a PT. The need to ensure adequate excision of the PT in some cases necessitates a mastectomy even when the carcinoma would be adequately treated by breast conservation.
OCCULT CARCINOMA PRESENTING WITH ALN METASTASES
Fewer than 1% of patients who have mammary carcinoma present with an ALN metastasis as the first clinical manifestation of the disease.129,130 Among 10,014 patients with primary operable breast carcinoma treated at one institution, 35 (0.35%) had occult carcinoma presenting with axillary metastases.
Clinical Presentation
This condition occurs throughout the entire age distribution of breast carcinoma,129,130,131,132,133,134,135 with the mean and median age around 57 years.136 The right axilla and breast were affected slightly more often (54%) than the left in one series,94 but others have reported left predominance.137,138,139 A positive family history of breast carcinoma has been reported in
nearly 50% of patients,133,135 with about 25% having a maternal first-degree relative affected.129
nearly 50% of patients,133,135 with about 25% having a maternal first-degree relative affected.129
The initial clinical presentation is enlargement of one or more ALNs. An abnormality may be reported on clinical examination of the ipsilateral breast in 25% of patients, but it is often not regarded as suspicious, or on follow-up it may not correlate with the location in the breast where carcinoma is ultimately detected.131,133,140 This observation is consistent with data compiled by Rosen et al.,141 who studied nearly 3,500 patients with palpable breast lesions and were studied by mammography. Carcinoma was diagnosed in 64 women. The palpable lesion proved to be carcinoma in 54 of these cases, but in 10 women the palpable tumor was benign, and carcinoma was a nonpalpable lesion detected by mammography alone. In this series, none of the patients was initially examined because of axillary nodal involvement, but the study demonstrated the capacity of mammography to detect clinically occult carcinoma in the presence of a benign, palpable mass.
Clinical Evaluation
To rule out an extramammary tumor or other metastases, most women have been studied with a variety of techniques.131,133,134 Marcantonio and Libshitz142 demonstrated ALN enlargement by computed tomography (CT) in patients with pulmonary carcinoma and proved the presence of metastatic carcinoma by biopsy in six cases, confirming the lung as one of the alternate primary sites for an occult carcinoma that presents with axillary metastases.
Mammography has revealed abnormalities in12%,131 25%,134 26.5%,129 31%,138 and 35%133,140 of patients examined. Tartter et al.143 compared women with false-negative and positive mammograms. The two groups were similar with respect to tumor differentiation, tumor size, and ER status. However, women with false-negative mammography had a lower frequency of intraductal carcinoma and significantly more frequent metastases in ALNs. Some investigators have excluded patients with significant mammographic abnormalities from the syndrome of subclinical carcinoma presenting with ALN metastases,132,144 but others found no consistent correlation between the location of the radiologic abnormality and the site at which a carcinoma was ultimately located.133 If mastectomy is delayed, repeat mammograms of patients who initially had negative studies may reveal new findings suggestive of carcinoma.140 In one study, the interval until the detection of a breast abnormality clinically or by mammography was 6 to 39 months, with a mean of 15 months in women who did not undergo a mastectomy.145 The presence of mammographically detectable calcifications in metastatic carcinoma in ALNs may be a clue to the diagnosis of a subclinical mammary carcinoma.146,147
MRI has proven to be an effective method for detecting occult carcinomas that are not evident mammographically. MRI detected occult carcinoma in 143 of 234 (61%) patients
in pooled results from 10 studies published until 2008.148 In another pooled study published in 2010, the specificity of MRI was 31% on pooled data (range, 22% to 50%) from seven studies.149 However, not all lesions detected by MRI in this setting prove to be carcinoma. Buchanan et al.136 reported false-positive MRI studies in 15 of 69 patients, and in another series, MRI yielded a false-positive result in 2 of 15 cases.150 The diagnostic yield is low in patients with a negative mammogram and a negative MRI,136 a situation that led the European Society of Breast Cancer Specialists to recommend that surgical treatment be avoided if MRI of the breast is negative.148 Positive MRI findings should be investigated by biopsy. In a high proportion of cases, lesions detected by mammography can be localized by sonography, making them amenable to sonographically directed needle core biopsy.151
in pooled results from 10 studies published until 2008.148 In another pooled study published in 2010, the specificity of MRI was 31% on pooled data (range, 22% to 50%) from seven studies.149 However, not all lesions detected by MRI in this setting prove to be carcinoma. Buchanan et al.136 reported false-positive MRI studies in 15 of 69 patients, and in another series, MRI yielded a false-positive result in 2 of 15 cases.150 The diagnostic yield is low in patients with a negative mammogram and a negative MRI,136 a situation that led the European Society of Breast Cancer Specialists to recommend that surgical treatment be avoided if MRI of the breast is negative.148 Positive MRI findings should be investigated by biopsy. In a high proportion of cases, lesions detected by mammography can be localized by sonography, making them amenable to sonographically directed needle core biopsy.151
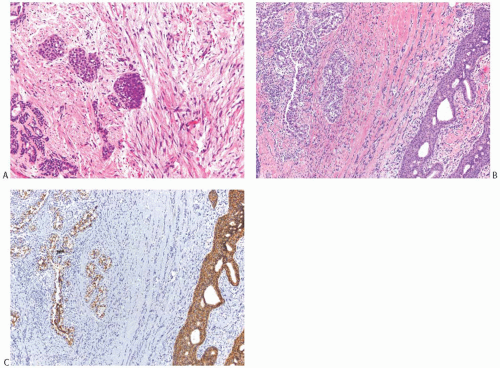
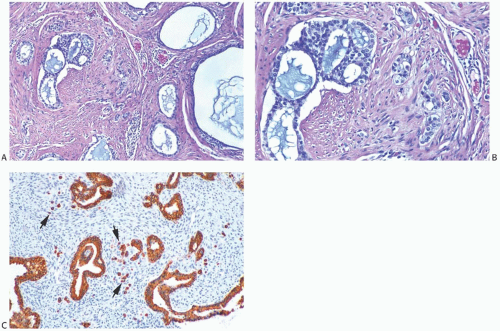 FIG. 33.9. Phyllodes tumor, benign, with intraductal and invasive ductal carcinoma. A,B: Cribriform intraductal carcinoma is next to IDC. C: Isolated cells (arrows) of IDC are highlighted by a CK immunostain in a benign PT. Glandular components of the PT are also cytokeratin positive (CK7). |
Occasionally, nodal enlargement occurs in the contralateral axilla of a patient treated previously for mammary carcinoma.139,152 This phenomenon was observed in 52 (3.6%) of 1,440 patients in one series.153




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree