Tubular Carcinoma
EDI BROGI
Tubular carcinoma is a distinct type of mammary carcinoma, first recognized nearly 150 years ago.1 The term “tubular” refers to the unique morphology of this tumor, which consists of simple neoplastic tubules lined by a single layer of cells with a structure that closely resembles normal mammary ductules.2,3
Pure tubular carcinoma constitutes less than 2% of all breast carcinomas.4,5,6,7,8,9,10,11 It tends to have small size and is relatively more frequent among T1 tumors. In one series, 5% of 382 T1N0M0 breast carcinomas were tubular.12 When further stratified by size, tubular carcinomas represented 9% of lesions 1.0 cm or smaller versus only 2% of carcinomas spanning 1.1 to 2.0 cm.12 Liu et al.13 reported that 97% of 71 tubular carcinomas were smaller than 2 cm (pT1). In a series by Rakha et al.,14 59% of 102 tubular carcinomas were smaller than 1 cm, 37% measured 1 to 2 cm, and only 4% were larger than 2 cm.
CLINICAL PRESENTATION
Imaging
Tubular carcinomas are often nonpalpable and are usually detected mammographically as irregular mass lesions. The conclusion that screening mammography has resulted in an increase in the frequency of this type of carcinoma being diagnosed is supported by many studies. Tubular carcinomas constituted 8% of invasive carcinomas spanning 1.0 cm or less detected in the Breast Cancer Detection Demonstration Projects.15 In one U.S. screening program, tubular carcinomas were 7% of 138 breast cancers; all tubular carcinomas were detected by mammography, but only 30% of them were evident clinically.16 A study correlating breast carcinoma histology and method of detection found that 83% of 41 tubular carcinomas had been detected mammographically, 12% were self-detected by the patient, and only 5% were found at clinical examination.17 Louwman et al.10 reviewed data for Dutch women aged 50 to 64 years with breast carcinoma diagnosed between 1988 and 2004. They found that tubular carcinoma constituted 8% of 9,259 screen-detected carcinomas, 2% of 5,413 interval carcinomas, and 2.1% of 6,710 carcinomas in women who did not undergo screening mammography. Similar results were obtained in Great Britain, where Nagtegaal et al.18 found that tubular carcinomas were 8.4% of screen-detected carcinomas, 2% of interval carcinomas, and 2.1% of carcinomas in women not participating in a mammographic screening program, respectively.
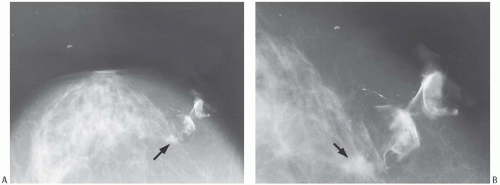
Mammographic findings can be suggestive of tubular carcinoma, but are not specific. The radiologic differential diagnosis of tubular carcinoma includes benign sclerosing lesions such as radial scar or sclerosing adenosis (SA), as well as well-differentiated invasive duct carcinoma. In one study, the average radiographic size of nonpalpable tubular carcinomas was 0.8 cm and that of palpable lesions 1.2 cm.19 Most tubular carcinomas are spiculated (Fig. 13.1), and they often harbor calcifications.20 Rounded lesions, densities with indistinct borders or calcifications in the absence of a mass rarely prove to be tubular carcinoma. Radiologically, tubular carcinoma and radial scar are nearly indistinguishable, as both lesions have similar growth patterns and tubular carcinoma can arise in a radial scar.21,22 Ultrasonography is also helpful in detecting tubular carcinoma, especially small lesions that are inapparent mammographically.23 No features specific for tubular carcinoma have been reported in magnetic resonance imaging studies.
Age
Among women, the age at diagnosis of tubular carcinoma ranges from 24 to 92 years.7,8,10,13,14,24,25,26,27,28 In one series,27 75% of tumors were diagnosed in women between ages 50 and
79 years. A study based on Surveillance Epidemiology and End Results (SEER) data for breast carcinomas reported that 73.6% of 4,477 tubular carcinomas diagnosed in the United States from 1992 and 2007 occurred in women 50 to 79 years old, 17.5% in women 40 to 49 years old, 6.9% in women older than
Family History
Tubular carcinoma was associated with a 40% frequency of positive family history of breast carcinoma among first-degree relatives in one study.32 This association may reflect selective factors related to the specific population studied, which included many women who had mammography performed because they had a positive family history. Others have not found a disproportionately high frequency of positive family history among relatives of women treated for tubular carcinoma.
Ethnicity and Menstrual Status
In a multicenter population-based case-control study conducted in five metropolitan areas of the United States, 80.5% of tubular carcinomas occurred in Caucasian/White women,33 and an epidemiologic study based on SEER data reported that 90% of patients with tubular carcinomas were non-Hispanic White women.11 The remaining patients were almost equally distributed, respectively, among women of African American (3.6%), Asian/Pacific Islander (3.5%), Hispanic White (2.3%), and American Indian/Alaska Native (0.5%) race or ethnicity.11
A family history of breast carcinoma in a first-degree relative tripled the risk of tubular carcinoma in premenopausal women, but was not significantly related to the occurrence of tubular carcinoma in postmenopausal patients.33 At least two studies have reported a two- to threefold increase in the risk of having tubular carcinoma in postmenopausal women who used hormone replacement therapy.33,34
Clinical Duration and Position in the Breast
Occasionally, a palpable lesion has reportedly been present for a substantial period before biopsy, or a relatively long duration can be established by retrospective review of mammograms, but the median duration prior to histologic diagnosis is about 2 months.24,25,26
Rarely, superficial tumors may be fixed to the skin, producing retraction signs. Tubular carcinoma usually occurs in peripheral portions of the breast, and nipple discharge is an infrequent presenting symptom.7 However, tubular carcinoma can arise near the major lactiferous ducts in the nipple or slightly lower in the subareolar region and raise the differential diagnosis of florid papillomatosis of the nipple. In this setting, the finding of Paget disease supports the diagnosis of tubular carcinoma (Fig. 13.2). Paget disease is very rarely found in association with tubular carcinoma not involving the nipple, except when coexistent DCIS separately involves the latter.
Stage and Lymph Node Metastases
A compilation of Netherlands registry data found that 70% of 3,456 patients with tubular carcinoma presented at stage I, 26% as stage II, 2% as stage III, and 1% as stage IV.10 According to SEER data, 90.5% of women diagnosed with tubular carcinoma presented at stage I, 8.9% at stage II, 0.4% at stage III, and 0.2% at stage IV.11
The average frequency of axillary lymph node (ALN) metastases resulting from tubular carcinoma is about 10%,4,7,12,24,25,26,35,36,37,38,39,40 ranging from none7,35 to 29%.21 A metaanalysis of 680 patients in published reports found axillary nodal metastases in 6.6% of those with pure tubular carcinoma and in 25% with “mixed” tubular carcinoma.41 Tubular carcinomas were only 1.5% of all carcinomas in a series of 142 patients with T1N1M0 disease.15
Affected lymph nodes are usually in the low axilla (level I), and only exceptionally are more than three lymph nodes involved.24 Fedko et al.42 found lymph node metastases in 5 (5.4%) of 93 patients with pure tubular carcinoma and known lymph node status: 2 patients had macrometastases, and the other 3 had micrometastases. Two additional patients had lymph nodes occupied by isolated tumor cells (N0(i+)).42 The size of the primary tumor ranged from 0.9 to 1.5 cm. Bradford et al.40 reported that 13.3% of patients with tubular carcinoma 1 cm or larger had axillary nodal metastases. On the other hand, Green et al.37 reported that three tubular carcinomas with nodal metastases were smaller than 1 cm, including one 0.4-mm lesion. In a retrospective analysis of sentinel lymph node (SLN) biopsy in 234 patients with tubular carcinoma treated at nine different French institutions,43 the procedure successfully identified one or more lymph nodes in 229/234 (98%) cases. Pathologic evaluation
of SLNs included routine stained sections and cytokeratin stains at three levels. Six of 234 (2.5%) patients had macrometastases, 15/234 (6.4%) had micrometastases, and 2/234 (0.8%) had isolated tumor cells (N0(i+)). The median size of the tubular carcinomas was 9.59 mm (range, 1 to 22). The median size of tumors with SLN metastases (either macroor micrometastases) was 12.17 versus 9.39 mm for tumors without lymph node metastases (p = 0.005). On multivariate analysis, pathologic tumor size greater than 10 mm was the only parameter significantly associated with lymph node metastases (p = 0.007). In this study, all patients with macrometastatic carcinoma had a tumor greater than 1 cm.
of SLNs included routine stained sections and cytokeratin stains at three levels. Six of 234 (2.5%) patients had macrometastases, 15/234 (6.4%) had micrometastases, and 2/234 (0.8%) had isolated tumor cells (N0(i+)). The median size of the tubular carcinomas was 9.59 mm (range, 1 to 22). The median size of tumors with SLN metastases (either macroor micrometastases) was 12.17 versus 9.39 mm for tumors without lymph node metastases (p = 0.005). On multivariate analysis, pathologic tumor size greater than 10 mm was the only parameter significantly associated with lymph node metastases (p = 0.007). In this study, all patients with macrometastatic carcinoma had a tumor greater than 1 cm.
GROSS PATHOLOGY
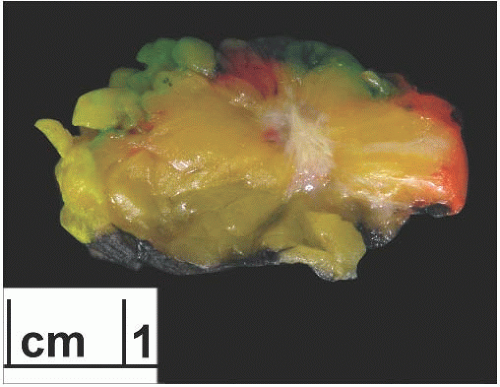
Grossly, tubular carcinoma forms an ill-defined firm to hard mass. When bisected, the lesion is often stellate and infiltrating, and the cut surface is likely to retract, becoming depressed in relation to the surrounding nonneoplastic tissue (Fig. 13.3). Most tumors are described as gray to white, but
lesions with extensive elastosis may appear tan or pale. Even if the gross appearance may suggest tubular carcinoma, microscopic examination is necessary to rule out a benign sclerosing lesion such as sclerosing papillomatosis with a radial scar pattern or an invasive ductal carcinoma of no special type.
lesions with extensive elastosis may appear tan or pale. Even if the gross appearance may suggest tubular carcinoma, microscopic examination is necessary to rule out a benign sclerosing lesion such as sclerosing papillomatosis with a radial scar pattern or an invasive ductal carcinoma of no special type.
Size
Most tubular carcinomas are 2 cm or less in diameter, but tumors as large as 4 cm have been described,4,6,7,24,25,26,44 and in one study the largest tumor spanned 12 cm.45 In some instances, tumors reported to be larger than 5 cm may be examples of coalescent multifocal lesions. Some investigators have reported that 67%27 to 87%24 of tubular carcinomas were 1 cm or smaller. In the latter study,24 the median size of 90 “pure” tubular carcinomas was 0.8 cm. Fifty-two percent of ductal carcinomas with tubular features were 1 cm or less. The median size of mixed duct and tubular lesions was 1.1 cm.
MICROSCOPIC PATHOLOGY
Microscopically, tubular carcinoma consists of a haphazard proliferation of small glands and tubules. The overall configuration tends to be stellate with ill-defined borders. Most of the glands and tubules are simple and lined by a single layer of neoplastic epithelium. “Tubularity” of a carcinoma can be expressed as the percentage of all tumor cells that directly line the lumens of the neoplastic glands.46 The diagnosis of tubular carcinoma is made when either the entire tumor or nearly all of it exhibits tubular growth pattern. Although there is no agreement on the lowest percentage of tubules required for the diagnosis of tubular carcinoma, 90% tubularity is commonly regarded as a “practical” cutoff. Focal multilayering of the epithelium or a more complex architecture are features compatible with the diagnosis of tubular carcinoma only if limited to a minority of the glands. When a tubular component involves less than 90% of a carcinoma, the lesion is often referred to as a “mixed” tubular carcinoma or ductal carcinoma with tubular features. These tumors form a very heterogeneous group with respect to the proportion of the tubular component and also in regard to the degree of differentiation of the nontubular element.
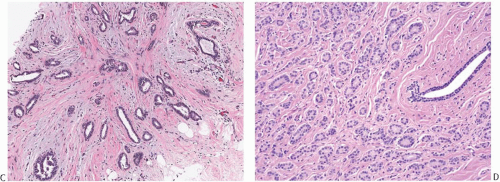
The glands of tubular carcinoma may have virtually any shape, but irregular shapes and angular contours predominate (Fig. 13.4). They usually have a widely patent lumen that is best appreciated at low-power examination. The neoplastic epithelium is cuboidal or columnar and has round to oval nuclei that tend to be basally oriented and show low-grade atypia. The neoplastic cells are usually homogeneous within a given lesion, but scattered glands show some variation in the height of the lining epithelium, with nearly flat epithelium adjacent to elongated columnar cells (Fig. 13.5). The cytoplasm is usually amphophilic or infrequently clear. It is commonly quite abundant, but can be scant. Cytoplasmic tufts or “snouts” are often present at the luminal cell border (Fig. 13.5). Eosinophilic cytoplasm and apical intracytoplasmic granules characteristic of apocrine differentiation are infrequent in a pure tubular carcinoma. Nucleoli are inconspicuous or inapparent, often somewhat peripheral in the nucleus and adjacent to the nuclear membrane. Mitoses are rarely seen, and necrosis is absent. Very uncommon variants of tubular carcinoma feature mucin secretion or apocrine differentiation47 (Fig.13.6).
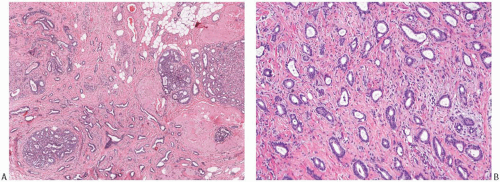 FIG. 13.4. Tubular carcinoma, various glandular patterns. A: Medium-power view of tubular carcinoma infiltrating between lobules involved by SA. The neoplastic glands have open lumens, in contrast to the compressed lumens of the tubules of SA. B: Oval and angular glands in moderately cellular stroma. C: Predominantly angular glands in core biopsy material. D: Round glands. This pattern resembles MGA. |
The stroma between the glands of tubular carcinoma often differs from the stroma in the surrounding nonneoplastic breast tissue, providing a subtle but useful clue to the presence of tubular carcinoma at low-power examination. The stroma admixed with tubular carcinoma is usually rich in myofibroblasts, abundant elastic tissue, and myxoid matrix48,49 (Figs. 13.4 and 13.5); it tends to be abundant and separates the neoplastic glands more widely than in nontubular well-differentiated ductal carcinomas. Elastosis has been regarded as a hallmark of tubular carcinoma (Fig. 13.5),48,49 but it is not present in all cases and can also be a prominent feature of nontubular carcinomas and of some benign lesions, particularly those with the “radial scar” pattern.
Calcifications are detected microscopically in at least 50% of tubular carcinomas. They may be present in the lumen of the neoplastic glands (Fig. 13.5) and in the stroma admixed with the tumor, as well as in the associated intraductal carcinoma component and in adjacent glands with low-grade atypia.
Tubular carcinoma does not elicit a notable lymphocytic reaction. Perineural invasion is uncommon (Fig. 13.7). Blood vessel invasion and lymphatic tumor emboli are virtually never seen except after needling procedures (Fig. 13.8).
Precursor Lesions Associated with Tubular Carcinoma
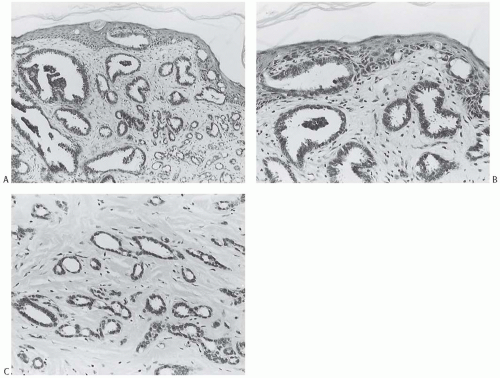
Ductal carcinoma in situ (DCIS) has been described in 21% to 84% of tubular carcinomas.6,24,25,38,50 The DCIS typically has papillary, micropapillary, or cribriform patterns or shows a mixed pattern24 (Figs. 13.9 and 13.10). In a significant number of cases, the intraductal proliferation associated with tubular carcinoma, and from which carcinoma appears to have arisen, is histologically indistinguishable from atypical ductal hyperplasia (ADH). Tubular carcinoma may arise from or in association with a benign proliferative lesion with a radial scar configuration.51,52
Tubular carcinoma frequently develops in breast tissue involved by a multifocal alteration of the terminal ductallobular units usually referred to as “columnar cell change (CCC)” (Figs. 13.9 and 13.10). This lesion was previously designated informally as “pretubular” hyperplasia.53 The spectrum of columnar cell lesions ranges from CCC and columnar cell hyperplasia (CCH) without atypia through CCC and/or hyperplasia with atypia to DCIS. Atypical CCH is also referred to as “flat epithelial atypia” (FEA). Cystic dilatation of the involved acini is a characteristic feature of these lesions, and Goldstein and O’Malley44 referred to carcinoma in this setting as “cancerization of small ectatic ducts of the breast by DCIS cells with apocrine snouts.” Cytomorphologic features common to all phases of CCC with atypia are nuclear hyperchromasia and a high nuclear-to-cytoplasmic ratio. Apical snouts are also frequently present at the luminal borders of ducts and ductules. The most subtle expression of this condition consists of a monostratified layer of cuboidal or low columnar cells, with the foregoing cytologic features. CCH is characterized by crowding of cells that become increasingly compressed and columnar, and display haphazard arrangement of the nuclei with respect to the basement membrane. Atypical CCH shows low-grade atypia with round to oval nuclei with smooth nuclear contour and finely dispersed homogeneous chromatin. Rare inconspicuous nucleoli are often seen abutting the nuclear membrane. The presence of blunt micropapillae or cribriform growth composed of the same proliferative cells with “streaming” or condensation of cells toward the lumen is often associated with atypical CCH, transitioning into ADH. The acini affected by CCCs may contain dense intraluminal flocculent secretions that often undergo calcification, forming small basophilic deposits. These laminated concretions are often detected mammographically as fine punctate clustered calcifications of indeterminate significance. DCIS with low nuclear grade and cribriform or micropapillary architecture often arises in the background of CCCs, whereas high-grade DCIS is extremely rare in this setting.54,55
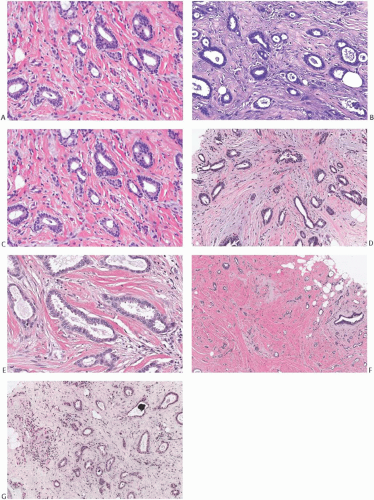 FIG. 13.5. Tubular carcinoma. A,B: Round or oval, orderly carcinomatous glands shown here resemble glands found in microglandular adenosis [MGA]. Note evidence of myofibroblastic proliferation in the stroma and stromal elastosis, which is not a feature of MGA. C,D: The glandular epithelium has uneven height, with flattened cells adjacent to columnar cells in adjacent tubules and even within the same gland. E: Some neoplastic glands have bulbous projections of the apical cytoplasm, often referred to as “apical snouts.” F: Stromal elastosis is evident in a tubular carcinoma with MGA-like glands. G: Minute calcifications are present in the lumens of neoplastic glands. |
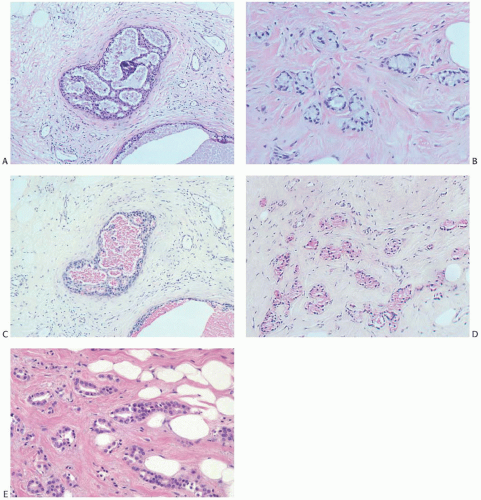 FIG. 13.6. Tubular carcinoma, unusual variants. A-D: Mucin secreting. A: DCIS associated with the tubular carcinoma. B: The invasive glands have clear cytoplasm and resemble MGA. C,D: Mucin in the intraductal and infiltrating carcinoma stains pink with the mucicarmine stain. E: Apocrine type. Tubular carcinoma glands with apocrine cytology. |
Columnar cell lesions are associated with classical lobular carcinoma in situ (LCIS) (Figs. 13.9 and 13.10), as well as with tubular carcinoma and tubulolobular carcinoma (described later).53,56,57 This complex has been referred to as the “Rosen triad.”58 In one study,50 8/14 (57%) tubular carcinomas had associated CCCs with atypia (FEA), 7/14 (50%) had micropapillary ADH, 3/14 (21%) had low nuclear grade DCIS




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree