Fig. 28.1 Anatomy of the plantar fascia. (a) The plantar fascia extends from the heal to the base of all toes. (b) Superficial muscles of the foot after the plantar fascia is removed. Flexor digitorum brevis lies directly beneath the fascia at the middle of the foot. Abductor hallucis and abductor digiti minimi are seen close to the heal on either side of flexor digitorum brevis.
Rationale for using botulinum neurotoxin for treatment of plantar fasciitis
The data from animal studies and early observations of pain relief with onabotulinumtoxinA (Botox in cervical dystonia) prompted investigations on the analgesic effect of botulinum neurotoxin (BoNT) in human pain disorders. In the rat formalin model, injection of onabotulinumtoxinA into the paw a week before formalin injection significantly reduced the inflammatory peak of pain and accumulation of glutamate locally (Cui et al., 2004). A number of other mechanisms also are described based on animal investigations (Aoki and Francis, 2011). In humans, BoNT treatment of chronic pain of cervical dystonia and migraine are approved by US Food and Drug Administration and double-blind, placebo-controlled studies indicate probable efficacy (class B evidence) in postherpetic and post-traumatic neuralgia, piriformis syndrome and total knee arthroplasty (Jabbari and Machado, 2011).
Randomized, prospective studies of botulinum neurotoxin treatment
At the present time, based on two class II, placebo-controlled, double-blind studies, onabotulinumtoxinA is considered “probably effective” in treatment of PF, meeting level B evidence according to the criteria of the American Academy of Neurology (French and Gronseth, 2008). In the first study (Babcock et al., 2005), 27 patients were randomized into group A (toxin) and group B (placebo). The onabotulinumtoxinA solution was prepared by mixing 100 U with 1 ml of preservative-free normal saline. Patients in group A received 70 U (0.7 ml) in two divided doses: 40 U (0.4 ml) in the tender region of the heel medial to the base of the plantar fascia insertion and 30 U (0.3 ml) in the most tender point of the arch of the foot (between the heel and middle of the foot) (Fig. 28.2). A 27-gauge, 20 mm (0.75 inch) needle was used for injections. Group B received normal saline at the same locations and of similar volume. Pain relief was measured by visual analog scale (VAS), pressure algometry and the Maryland foot score at baseline (before treatment) and at 3 and 8 weeks after injection. Twenty seven patients participated in the study (11 with unilateral and 16 with bilateral PF). In patients with bilateral PF, one foot received onabotulinumtoxinA and the other placebo. The pain response to BoNT compared with placebo was statistically significant at both 3 and 8 weeks (p < 0.005, <0.001, <0.0003 for VAS, pressure algometry and Maryland foot score, respectively). None of the patients reported any side effects.
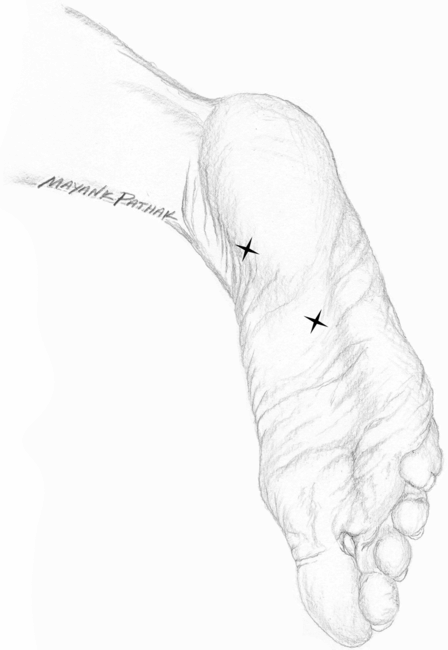
Fig. 28.2 Injection sites in plantar fasciitis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


