Chapter 30 Treatment of Affective Disorders
| Abbreviations | |
|---|---|
| DA | Dopamine |
| 5-HT | Serotonin |
| MAO | Monoamine oxidase |
| MAOI | Monoamine oxidase inhibitor |
| NE | Norepinephrine |
| SNRI | Serotonin/norepinephrine reuptake inhibitor |
| SSRI | Serotonin selective reuptake inhibitor |
| TCA | Tricyclic antidepressant |
Therapeutic Overview
changes characterized by severe highs and gut-wrenching lows, which may worsen to a psychotic state. In addition, depression is often associated with comorbid anxiety disorders.
Although the molecular and cellular etiology of depression remains unknown, it is generally accepted that depression involves impaired monoaminergic neurotransmission, leading to alterations in the expression of specific genes. This is supported by studies demonstrating that antidepressants increase the expression of the transcription factor cyclic adenosine monophosphate response element-binding protein (CREB) and brain-derived neurotrophic factor (BNDF), both of which are critical for maintaining normal cell structure in limbic regions of the brain that are targets for monoaminergic projections. In addition, postmortem and imaging studies have demonstrated neuronal loss and shrinkage in the prefrontal cortex and hippocampus in depressed patients, some of which could be reversed by antidepressants.
The treatment of bipolar disorder has changed over the past decade. Lithium has been the mainstay of treatment for many years, particularly for control of the manic phase. However, the anticonvulsants lamotrigine, valproic acid, and carbamazepine have been frequently used as well (see Chapter 34), especially in cases in which the bipolar disorder was characterized by rapid cycling. Recently, the atypical antipsychotic drugs aripiprazole, olanzapine, quetiapine, risperidone, and ziprasidone were approved as monotherapy for bipolar disorder (see Chapter 29). Antidepressants may also be warranted to treat the depressive phase of the illness.
The pharmacology of the antipsychotics is discussed in Chapter 29 and that of the anticonvulsants in Chapter 34. Therapeutic actions related to the antidepressants and lithium are summarized in the Therapeutic Overview Box.
Mechanisms of Action
Amine Reuptake Inhibitors and Atypical Antidepressants
| Therapeutic Overview |
|---|
| Antidepressants |
| Prolong the action of biogenic amines at the synapse by inhibiting amine reuptake, increasing amine release or decreasing amine catabolism |
| Enhance neurogenesis and dendritic branching in the adult hippocampus |
| Lithium |
| Interferes with receptor-activated phosphatidylinositol turnover; blocks the conversion of inositol phosphate to free inositol |
| Antagonizes 5-HT1A and 5-HT1B autoreceptors, alleviating feedback inhibition of 5-HT release |
| Enhances glutamate reuptake system, clearing glutamate from the synapse |
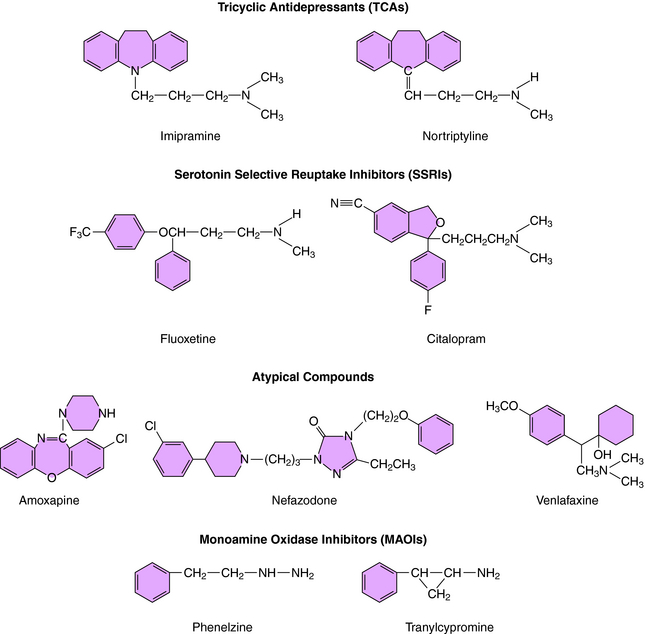
was the first agent demonstrated to have antidepressant efficacy. The TCAs have a three-ring structure with a side chain containing a tertiary or secondary amine attached to the central ring, resembling the phenothiazine antipsychotics (Fig. 30-1). The tertiary amines include imipramine, amitriptyline, clomipramine, and doxepin; the secondary amines include desipramine and nortriptyline.
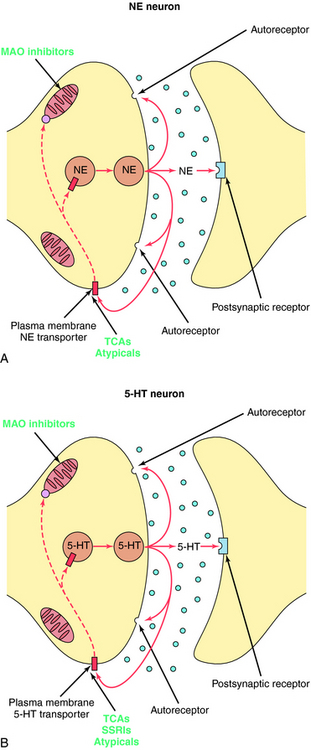
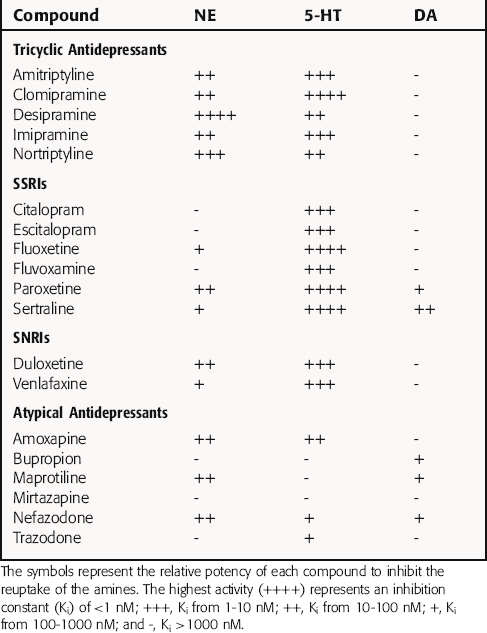
The TCAs block the reuptake of NE, 5-HT, or both into noradrenergic and/or serotonergic nerve terminals, respectively, by specific interactions with plasma membrane transporters (Fig. 30-2). As a consequence of this inhibition, the actions of NE and 5-HT released from these neurons are not rapidly terminated, resulting in a prolonged stimulation of NE receptors, 5-HT receptors, or both. The TCAs do not affect the reuptake of DA by dopaminergic nerve terminals, and their selectivity for NE versus 5-HT transporters differs among the different compounds (Table 30-1).
The SSRIs and SNRIs also inhibit the reuptake of biogenic amines, and as their name implies, the SSRIs have the highest affinity for 5-HT transporters, whereas the SNRIs have high affinity for 5-HT transporters and moderate affinity for NE transporters. It is important to note, however, that specificity and selectivity are always dose-related such that the SSRIs sertraline and paroxetine inhibit both NE and DA reuptake at the upper end of their dose ranges (see Table 30-1). Similarly, it is also important to keep in mind that the classification of newly developed compounds is based on their affinity for specific transporters, whereas that of the TCAs is based on chemical structure. Thus, although clomipramine is classified chemically as a TCA, its ability and selectivity to inhibit 5-HT and NE reuptake matches that of the SSRI paroxetine. Likewise, the selectivity of the TCAs imipramine and amitriptyline resemble that of the SNRI duloxetine. Thus, at times, classification schemes may be misleading.
As mentioned, the atypical compounds are a very heterogeneous group of drugs. Among these, maprotiline and nefazodone are relatively selective inhibitors of NE reuptake (see Table 30-1). Trazodone is a weak inhibitor of 5-HT reuptake, bupropion weakly inhibits DA reuptake, and mirtazapine appears devoid of activity at any reuptake transporter.
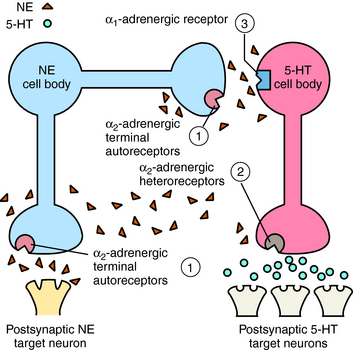
Mirtazapine also blocks α2 adrenergic receptors on noradrenergic and serotonergic nerve terminals and on noradrenergic dendrites (Fig. 30-3). Stimulation of α2 autoreceptors on noradrenergic neurons decreases NE release, whereas stimulation of α2 heteroreceptors on serotonergic neurons inhibits 5-HT release. In addition, stimulation of α1 adrenergic receptors on serotonergic cell bodies and dendrites increases their firing rate. Thus mirtazapine, by inhibiting α2 autoreceptors, enhances noradrenergic cell firing and the release of NE, which activates α1 adrenergic receptors to increase 5-HT release while concurrently blocking α2 heteroreceptors, further facilitating the release of 5-HT.
The MAOIs used for the treatment of depression are phenelzine and tranylcypromine (see Fig. 30-1) and the recently approved selegiline transdermal patch. Phenelzine and selegiline are irreversible MAO inhibitors, and tranylcypromine is a long-lasting MAO inhibitor. At the doses used for depression, all these compounds are nonselective and inhibit both MAO-A and MAO-B. These enzymes are distinct gene products with MAO-A present in human placenta, intestinal mucosa, liver, and brain—responsible for the catabolism of 5-HT, NE, and tyramine; and MAO-B present in human platelets, liver, and brain—responsible predominantly for the catabolism of DA and tyramine. These enzymes are located in the outer membrane of mitochondria and function to maintain low cytoplasmic concentrations of the monoamines, facilitating inward-directed transporter activity (i.e., monoamine reuptake). MAO inhibition causes an increase in monoamine concentrations in the cytosol of the nerve terminal. All the effects of the MAOIs have been attributed to enhanced aminergic activity resulting from enzyme inhibition.
Research with selective MAOIs has shown that inhibition of MAO-A is necessary for antidepressant activity. Thus, although selegiline is a selective inhibitor of MAO-B at low doses and is used at these doses for the treatment of Parkinson’s disease (see Chapter 28), at higher doses selectivity is lost, and selegiline inhibits both MAO-A and MAO-B and has antidepressant activity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree