CHAPTER 31 Transport and Metabolic Functions of the Liver
OVERVIEW OF THE LIVER AND ITS FUNCTIONS
The liver is a large, multilobed organ located in the abdominal cavity whose function is intimately associated with that of the gastrointestinal system. The liver serves as the first site of processing for most absorbed nutrients and also secretes bile acids, which as we learned in Chapter 29, plays a critical role in the absorption of lipids from the diet. In addition, the liver is a metabolic powerhouse, critical for disposing of a variety of metabolic waste products and xenobiotics from the body by converting them to forms that can be excreted. The liver stores or produces numerous substances needed by the body, such as glucose, amino acids, and plasma proteins. In general, key functions of the liver can be divided into three areas: (1) contributions to whole-body metabolism, (2) detoxification, and (3) excretion of protein-bound/lipid-soluble waste products. In this chapter we discuss the structural and molecular features of the liver and the biliary system that subserve these functions, as well as their regulation. However, although the liver contributes in a pivotal way to the maintenance of whole-body biochemical status, a complete discussion of all of the underpinning reactions is beyond the scope of the present text. We will confine our discussion primarily to hepatic functions that relate to gastrointestinal physiology.
Metabolic Functions of the Liver
The liver also plays a vital role in protein metabolism. The liver synthesizes all of the so-called nonessential amino acids (see Chapter 29) that do not need to be supplied in the diet, in addition to participating in interconverting and deaminating amino acids so that the products can enter biosynthetic pathways for the synthesis of carbohydrates. With the exception of immunoglobulins, the liver synthesizes almost all of the proteins present in plasma, especially albumin, which determines plasma oncotic pressure, as well as most of the important clotting factors. Patients suffering from liver disease may develop peripheral edema secondary to hypoalbuminemia and are also susceptible to bleeding disorders. Finally, the liver is the critical site for disposal of the ammonia generated from protein catabolism. This is accomplished by converting ammonia to urea, which can then be excreted by the kidneys. The details of this process will be discussed later.
The Liver and Detoxification
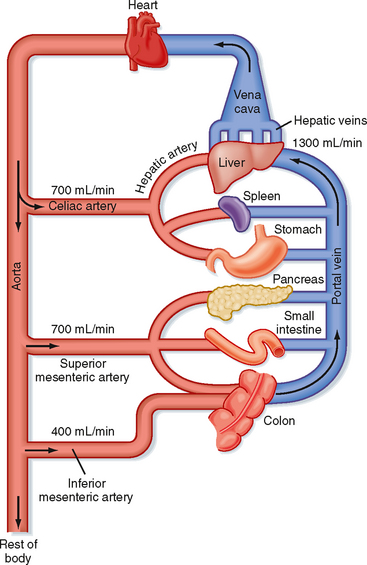
The liver serves both as a gatekeeper, by limiting the entry of toxic substances into the bloodstream, and as a garbage disposal, by extracting potentially toxic metabolic products produced elsewhere in the body and converting them to chemical forms that can be excreted. The liver fulfills these functions, in part, because of its unusual blood supply. Unlike all other organs, the majority of blood arriving at the liver is venous in nature and is supplied via the portal vein from the intestine (Fig. 31-1). As such, the liver is strategically located to receive not only absorbed nutrients but also potentially harmful absorbed molecules such as drugs and bacterial toxins. Depending on the efficiency with which these molecules are extracted by hepatocytes and subjected to so-called first-pass metabolism, little or none of the absorbed substance may make it into the systemic circulation. This is a major reason why not all pharmaceutical agents can achieve therapeutic concentrations in the bloodstream if administered orally.
Role of the Liver in Excretion
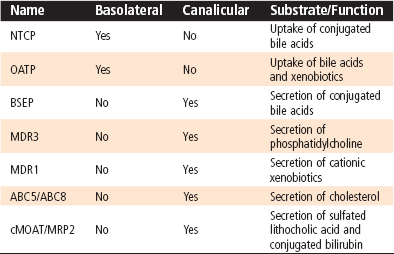
The kidneys play an important role in the excretion of water-soluble catabolites, as discussed in the renal section. Only relatively small water-soluble catabolites can be excreted by the process of glomerular filtration. However, larger water-soluble catabolites and molecules bound to plasma proteins, including lipophilic metabolites and xenobiotics, steroid hormones, and heavy metals, cannot be filtered by the glomerulus. All these substances are potentially harmful if allowed to accumulate, so a mechanism must exist for their excretion. The mechanism for excretion involves the liver, which excretes these substances in bile. Hepatocytes take up these substances with high affinity by virtue of the presence of an array of basolateral membrane transporters, and the substances are subsequently metabolized at the level of microsomes and in the cytosol (Table 31-1). Ultimately, substances destined for excretion in bile are exported across the canalicular membrane of hepatocytes via a different array of transporters. The features of bile allow solubilization of even lipophilic substances, which can then be excreted into the intestine and ultimately leave the body in feces.
STRUCTURAL FEATURES OF THE LIVER AND BILIARY SYSTEM
Hepatocytes, the major cell type in the liver, are arranged in anastomosing cords that form plates around which large volumes of blood circulate (Fig. 31-2). The liver receives a high blood flow that is disproportionate to its mass, which ensures that hepatocytes receive high quantities of both O2 and nutrients. Hepatocytes receive more than 70% of their blood supply at rest via the portal vein (rising to more than 90% in the postprandial period).
(Modified from Bloom W, Fawcett DW: A Textbook of Histology, 10th ed. Philadelphia, Saunders, 1975.)
The plates of hepatocytes that constitute the liver parenchyma are supplied by a series of sinusoids, which are low-resistance cavities supplied by branches of both the portal vein and the hepatic artery. The sinusoids are unlike the capillaries that perfuse other organs. During fasting, many sinusoids are collapsed, but more can gradually be recruited as portal blood flow increases during the period after a meal when absorbed nutrients are transported to the liver. The low resistance of the sinusoidal cavities means that blood flow through the liver can increase considerably without a concomitant increase in pressure. Eventually, the blood drains into central branches of the hepatic vein.
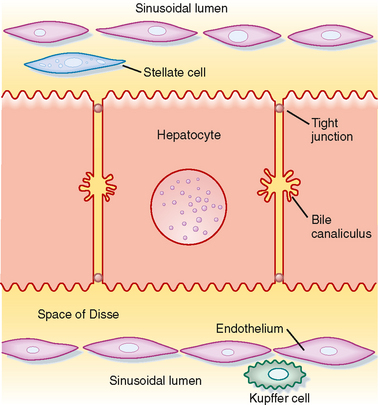
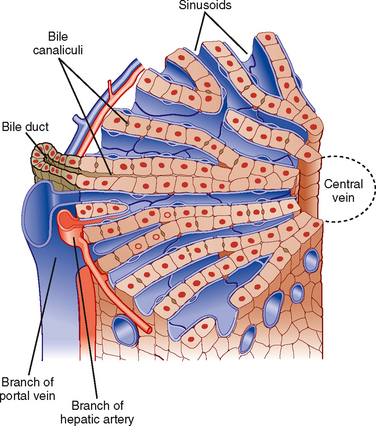
The sinusoids are also unusual in the endothelial cells that line their walls (Fig. 31-3). Hepatic endothelial cells contain specialized openings, known as fenestrations, that are large enough to permit the passage of molecules as big as albumin. Sinusoidal endothelial cells also lack a basement membrane, which might otherwise pose a diffusion barrier. These features allow access of albumin-bound substances to the hepatocytes that will eventually take them up. The sinusoids also contain Kupffer cells. Beneath the sinusoidal endothelium and separating the endothelium from the hepatocytes is a thin layer of loose connective tissue called the space of Disse, which likewise poses little resistance to the movement of molecules even as large as albumin in health. The space of Disse is also the location of another important hepatic cell type, the stellate cell. Stellate cells serve as storage sites for retinoids and in addition are the source of key growth factors for hepatocytes. Under abnormal conditions, stellate cells are activated to synthesize large quantities of collagen, which contributes to the hepatic dysfunction.
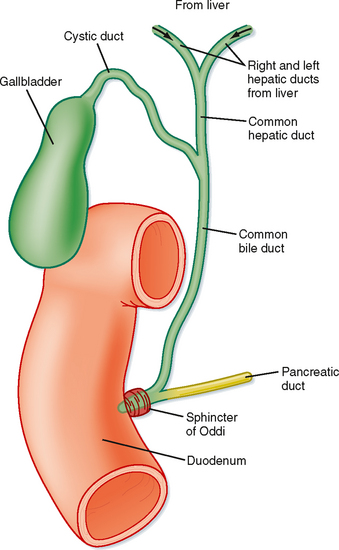
Hepatocytes are also the origination point for the biliary system. Although hepatocytes are considered to be epithelial cells with basolateral and apical membranes, the spatial arrangement of these two cell domains differs from that seen in simple columnar epithelium, such as that lining the gastrointestinal tract. Rather, in the liver the apical surface of the hepatocyte occupies only a small fraction of the cell membrane, and the apical membranes of adjacent cells oppose each other to form a channel between the cells known as the canaliculus (Fig. 31-3). The role of canaliculi is to drain bile from the liver, and these canaliculi drain into biliary ductules, which are lined by classic columnar epithelial cells known as cholangiocytes. Ultimately, the biliary ductules drain into large bile ducts that coalesce into the right and left hepatic ducts to permit exit of bile from the liver. These, in turn, form the common hepatic duct, from which bile can flow into either the gallbladder, via the cystic duct, or the intestine, via the common bile duct (Fig. 31-4), on the basis of prevailing pressure relationships.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree