CHAPTER 11 The Autonomic Nervous System and Its Central Control
The main function of the autonomic nervous system is to assist the body in maintaining a constant internal environment (homeostasis). When internal stimuli signal that regulation of the body’s environment is required, the central nervous system (CNS) and its autonomic outflow issue commands that lead to compensatory actions. For example, a sudden increase in systemic blood pressure activates the baroreceptors, which in turn modify the activity of the autonomic nervous system so that the blood pressure is restored toward its previous level (see Chapter 17).
The term autonomic nervous system generally refers to the sympathetic and parasympathetic nervous systems. In this chapter, the enteric nervous system is also included as part of the autonomic nervous system, although it is sometimes considered a separate entity (see also Chapter 32). In addition, because the autonomic nervous system is under CNS control, the central components of the autonomic nervous system are discussed in this chapter. The central components include the hypothalamus and higher levels of the limbic system, which are associated with emotions and with many visceral types of behavior (e.g., feeding, drinking, thermoregulation, reproduction, defense, and aggression) that have survival value.
ORGANIZATION OF THE AUTONOMIC NERVOUS SYSTEM
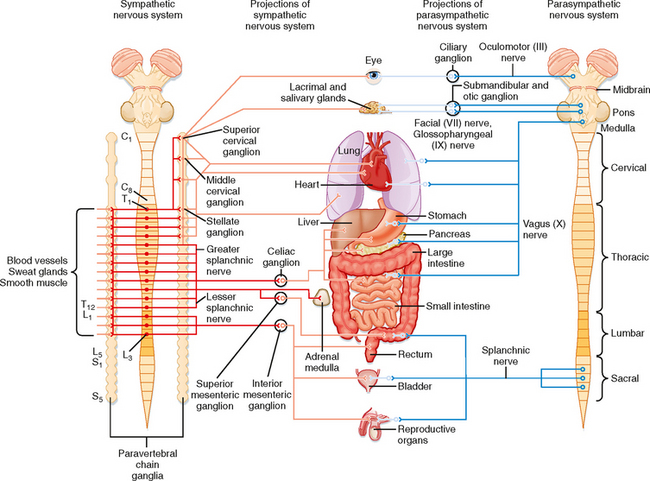
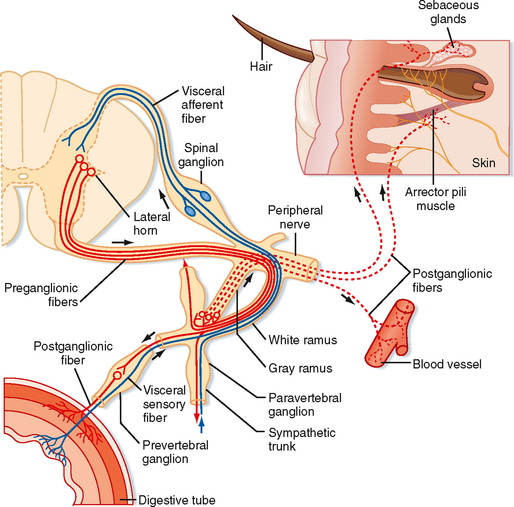
The primary functional unit of the sympathetic and parasympathetic nervous systems is the two-neuron motor pathway, which consists of a preganglionic neuron, whose cell body is located in the CNS, and a postganglionic neuron, whose cell body is located in one of the autonomic ganglia (Figs. 11-1 and 11-2). The enteric nervous system includes the neurons and nerve fibers in the myenteric and submucosal plexuses, which are located in the wall of the gastrointestinal tract.
The sympathetic preganglionic neurons are located in the thoracic and upper lumbar segments of the spinal cord. For this reason, the sympathetic nervous system is sometimes referred to as the thoracolumbar division of the autonomic nervous system. In contrast, the parasympathetic preganglionic neurons are found in the brainstem and in the sacral spinal cord. Hence, this part of the autonomic nervous system is sometimes called the craniosacral division. Sympathetic postganglionic neurons are generally found in the paravertebral or prevertebral ganglia. The paravertebral ganglia form two sets of ganglia, one lateral to each side of the vertebral column. Each set of ganglia is linked by longitudinally running axons to form a sympathetic trunk (Figs. 11-1 and 11-2). Prevertebral ganglia are located in the abdominal cavity (Fig. 11-1). Thus, paravertebral and prevertebral ganglia are located at some distance from their target organs. In contrast, parasympathetic postganglionic neurons are found in ganglia, which lie near or actually in the walls of the target organs.
The Sympathetic Nervous System
Sympathetic preganglionic neurons are concentrated in the intermediolateral cell column (lateral horn) in the thoracic and upper lumbar segments of the spinal cord (Fig. 11-2). Some neurons may also be found in the C8 segment. In addition to the intermediolateral cell column, groups of sympathetic preganglionic neurons are found in other locations, including the lateral funiculus, the intermediate gray matter, and the gray matter dorsal to the central canal.
The axons of preganglionic neurons are often small myelinated nerve fibers known as B fibers (see Table 5-1). However, some are unmyelinated C fibers. They leave the spinal cord in the ventral root and enter the paravertebral ganglion at the same segmental level through a white communicating ramus. White rami are found only from T1 to L2. The preganglionic axon may synapse on postganglionic neurons in this ganglion; may travel rostrally or caudally within the sympathetic trunk and give off collaterals to the ganglia that it passes; or may pass through the ganglion, exit the sympathetic trunk, and enter a splanchnic nerve to travel to a prevertebral ganglion (Figs. 11-1 and 11-2). A splanchnic nerve is a nerve that innervates the viscera; it contains both visceral afferents and autonomic fibers (sympathetic or parasympathetic).
Postganglionic neurons whose somata lie in paravertebral ganglia generally send their axons through a gray communicating ramus to enter a spinal nerve (Fig. 11-2). Each of the 31 pairs of spinal nerves has a gray ramus. Postganglionic axons are distributed through the peripheral nerves to effectors, such as piloerector muscles, blood vessels, and sweat glands, located in the skin, muscle, and joints. Postganglionic axons are generally unmyelinated (C fibers), although some exceptions exist. The distinction between white and gray rami is a consequence of the relative content of myelinated and unmyelinated axons in these rami.
The Parasympathetic Nervous System
The parasympathetic preganglionic neurons are located in several cranial nerve nuclei in the brainstem, as well as in the intermediate region of the S3 and S4 segments of the sacral spinal cord (Fig. 11-1). The cranial nerve nuclei that contain parasympathetic preganglionic neurons are the Edinger-Westphal nucleus (cranial nerve III), the superior (cranial nerve VII) and inferior (cranial nerve IX) salivatory nuclei, and the dorsal motor nucleus and nucleus ambiguus (cranial nerve X). Postganglionic parasympathetic cells are located in cranial ganglia, including the ciliary ganglion (preganglionic input is from the Edinger-Westphal nucleus), the pterygopalatine and submandibular ganglia (input from the superior salivatory nucleus), and the otic ganglion (input from the inferior salivatory nucleus). The ciliary ganglion innervates the pupillary sphincter and ciliary muscles in the eye. The pterygopalatine ganglion supplies the lacrimal gland, as well as glands in the nasal and oral pharynx. The submandibular ganglion projects to the submandibular and sublingual salivary glands and to glands in the oral cavity. The otic ganglion innervates the parotid salivary gland and glands in the mouth.
The parasympathetic preganglionic neurons that project to the viscera of the thorax and part of the abdomen are located in the dorsal motor nucleus of the vagus (see Fig. 4-7 E, F) and the nucleus ambiguus. The dorsal motor nucleus is largely secretomotor (it activates glands), whereas the nucleus ambiguus is visceromotor (it modifies the activity of cardiac muscle). The dorsal motor nucleus is secretomotor (it activates glands) and visceromotor (it activates the smooth muscle of the gut), whereas the nucleus ambiguus is visceromotor (it modifies the activity of cardiac muscle). Electrical stimulation of the dorsal motor nucleus results in gastric acid secretion, as well as secretion of insulin and glucagon by the pancreas. Although projections to the heart have been described, their function is uncertain. The nucleus ambiguus contains two groups of neurons: (1) a dorsal group (branchiomotor) that activates striated muscle in the soft palate, pharynx, larynx, and esophagus and (2) a ventrolateral group that innervates and slows the heart (see also Chapter 18).
Visceral Afferent Fibers
Visceral afferent fibers that mediate sensation include nociceptors that travel in sympathetic nerves, such as the splanchnic nerves. Visceral pain is caused by excessive distention of hollow viscera, contraction against an obstruction, or ischemia. The origin of visceral pain is often difficult to identify because of its diffuse nature and its tendency to be referred to somatic structures (see Chapter 7). Visceral nociceptors in sympathetic nerves reach the spinal cord via the sympathetic chain, white rami, and dorsal roots. The terminals of nociceptive afferent fibers project to the dorsal horn and to the region surrounding the central canal. They activate not only local interneurons, which participate in reflex arcs, but also projection cells, which include spinothalamic tract cells that signal pain to the brain.
Other visceral afferent fibers travel in parasympathetic nerves. These fibers are generally involved in reflexes rather than sensation (except for taste afferent fibers; see Chapter 8). For example, the baroreceptor afferent fibers that innervate the carotid sinus are in the glossopharyngeal nerve. They enter the brainstem, pass through the solitary tract, and terminate in the nucleus of the solitary tract (see Fig. 4-7, D—F). These neurons connect with interneurons in the brainstem reticular formation. The interneurons, in turn, project to the autonomic preganglionic neurons that control heart rate and blood pressure (see Chapter 18).
The Enteric Nervous System
The enteric nervous system, which is located in the wall of the gastrointestinal tract, contains about 100 million neurons. The enteric nervous system is subdivided into the myenteric plexus, which lies between the longitudinal and circular muscle layers of the gut, and the submucosal plexus, which lies in the submucosa of the gut. The neurons of the myenteric plexus primarily control gastrointestinal motility (see Chapter 26), whereas those in the submucosal plexus primarily regulate body fluid homeostasis (see Chapter 34).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree