Chapter 20 Suzanne A. Arinsburg; Joseph (Yossi) Schwartz; Eldad A. Hod; Richard O. Francis; Jeffrey S. Jhang; Yvette C. Tanhehco; Steven L. Spitalnik B. Erythrocytapheresis. C. Thrombocytapheresis. D. Plasmapheresis. E. Low-density lipoprotein (LDL) apheresis. 2a. The instrument shown in Figure 20-1 is being used to perform which one of the following procedures? B. Plateletpheresis. C. Red blood cell (RBC) exchange. D. Leukapheresis. E. Stem cell collection. 2b. Which labeled container in Figure 20-1 contains the most fibrinogen? B. B C. C D. D E. None of the labeled containers contains measurable amounts of fibrinogen. 3. Which statement best describes the use of therapeutic plasma exchange (TPE) for the desensitization of renal transplant candidates with a positive living donor kidney crossmatch due to donor-specific human leukocyte antigen (HLA) antibodies? A. TPE is considered to be first-line therapy. B. TPE is considered to be second-line therapy. C. The benefit of TPE is unclear. D. TPE is ineffective. E. TPE has never been tried in this setting. 4. A 53-year-old 70-kg man with a history of acute myelogenous leukemia (AML) is admitted with shortness of breath and headache and a white blood cell (WBC) count of 120 × 109/L (reference range 3.54 to 9.06 × 109/L), hemoglobin of 6.5 g/dL (reference range 13.3 to 16.2 g/dL), and a platelet count of 50 × 109/L (reference range 165 to 415 × 109/L). The procedure shown in Figure 20-2 is initiated. Which one of the following choices is the most appropriate volume of blood to be processed? B. 5 to 7.5 L. C. 7.5 to 10 L. D. 10 to 15 L. E. 15 to 20 L. 5. A 45-year-old man with sickle cell anemia is scheduled to undergo knee replacement surgery. His preoperative hemoglobin level is 6.0 g/dL (reference range 13.3 to 16.2 g/dL) with a hemoglobin S level of 80%. He is currently not receiving any therapy for sickle cell anemia. Which one of the following preoperative management options is indicated? A. No specific therapy is indicated. B. Begin hydroxyurea and delay surgery until the maximum tolerated dose (MTD) is achieved. C. Transfuse 4 U of RBCs. D. Perform an exchange transfusion, setting the end hemoglobin to 10.0 g/dL and the fraction of cells remaining to 0.3. E. Perform an exchange transfusion, setting the end hemoglobin to 10.0 g/dL and the fraction of cells remaining to 0.6. 6. A 22-year-old patient with sickle cell anemia receives an exchange transfusion with 4 U banked RBCs. Pre- and postprocedure hemoglobin quantification by high-performance liquid chromatography (HPLC) is shown in Figure 20-3 and Table 20-1. Hemoglobin quantification from one of the units the patient received is shown. Hemoglobin Hasharon has a retention time of 4.77 minutes. In which one of the following scenarios would transfusion of the implicated unit shown in the figure result in the least amount of circulating hemoglobin Hasharon in the patient after the procedure? A. The unit was the first of the four used for the exchange. B. The unit was the second of the four used for the exchange. C. The unit was the third of the four used for the exchange. D. The unit was the fourth of the four used for the exchange. E. The order of use of the units would not affect the postprocedure percentage of circulating hemoglobin Hasharon. 7. A 13-year-old 50-kg boy with sickle cell anemia is admitted to your hospital with cough, headache, wheezing, O2 saturation of 70% on nonrebreather mask, and multilobar pulmonary infiltrates on chest radiograph. The patient is in the intensive care unit (ICU) and is about to be intubated. His hemoglobin level is 8.5 g/dL (reference range 12.8 to 16.0 g/dL) with a hemoglobin S level of 80%. What is the optimal management option if available immediately? B. Hydroxyurea 20 mg/kg. C. Manual exchange using 6 U RBCs. D. Automated RBC exchange (RCE) transfusion, setting the end hematocrit to 30% and the fraction of RBCs remaining to 0.3. E. Partial automated RCE transfusion, setting the end hemoglobin to 12.0 g/dL and the fraction of cells remaining to 0.6. 8. Therapeutic apheresis is a first-line treatment for which one of the following disorders? A. Warm autoimmune hemolytic anemia. B. Hyperviscosity with monoclonal gammopathy. C. Bleeding with coagulation factor inhibitors. D. Thrombosis with antiphospholipid antibody syndrome. E. Bleeding with posttransfusion purpura. 9. Which one of the following rationales for use of therapeutic leukocytapheresis in the treatment of hyperleukocytosis associated with leukemia is best supported by current evidence? A. To improve the efficacy of chemotherapy by decreasing the overall tumor burden. B. To improve long-term mortality in patients with acute myeloid leukemia. C. To prevent leukostasis and the resultant complications in patients with chronic myelogenous leukemia. D. To treat complications associated with leukostasis in patients with acute myeloid leukemia. E. To prevent tumor lysis syndrome in patients with acute lymphoblastic leukemia. 10. RBC exchange (RCE) is a category II indication for the treatment of severe malaria. Which one of the following statements regarding RCE for the treatment of malaria is true? B. Antimalarial therapy should be held until after the RCE is completed. C. There is a high risk of RBC antigen alloimmunization in these patients and RCE should be avoided. D. A minimum of two RCEs should be performed to decrease the parasitemia significantly. E. RCE is used only for severe malaria in conjunction with antimalarial therapy. 11. Which one of the following patients is the best candidate for therapeutic plateletpheresis based on current evidence? B. A 20-year-old man status post-splenectomy for a traumatic splenic laceration with a platelet count of 1000 × 109/L. C. A 45-year-old woman with ovarian carcinoma, a history of thromboembolism, and a platelet count of 750 × 109/L. D. A 70-year-old man with chronic myelogenous leukemia, a history of myocardial infarction, and a platelet count of 750 × 109/L. E. A 57-year-old woman with chronic myelogenous leukemia and acute hemorrhage, and a platelet count of 750 × 109/L. 12. The American Society for Apheresis (ASFA) periodically publishes guidelines, using an evidence-based approach, regarding the use of therapeutic apheresis in clinical practice. Based on the 2013 ASFA guidelines, there are four categories describing the indications for therapeutic apheresis. Which one of the following is the best description of a category III indication by the ASFA 2013 guidelines? B. Disorders for which apheresis is accepted as second-line therapy, either as a stand-alone treatment or in conjunction with other modes of treatment. C. The optimal role of apheresis is not established; decision making should be individualized. D. Disorders in which published evidence demonstrates or suggests that apheresis is ineffective or harmful. Institutional review board (IRB) approval is desirable if apheresis treatment is undertaken in these circumstances. E. Disorders that, based on the available evidence, should never be treated with apheresis. 13. Thrombotic thrombocytopenic purpura (TTP) is a category I indication for TPE. Which one of the following statements best describes the rationale behind its efficacy? B. TPE removes B cells from the circulation, thereby decreasing ADAMTS13 autoantibody production. C. TPE removes ADAMTS13 autoantibody and replaces ADAMTS13 protease activity. D. TPE removes ADAMTS13 protease activity and replaces platelets. E. TPE provides ultra-large von Willebrand multimers to the patient. 14. Fresh frozen plasma (FFP) is an appropriate replacement fluid during therapeutic apheresis for which one of the following indications? A. Renal transplantation with antibody-mediated rejection. B. Acute chest syndrome in sickle cell disease. C. Acute inflammatory demyelinating polyradiculopathy (i.e., Guillain-Barré syndrome). D. Phytanic acid storage disease (i.e., Refsum disease). E. TTP. 15. Excellent vascular access is important during apheresis procedures due to the high-flow rates required by the instrumentation. Which one of the following statements is true? A. Femoral venous access is preferred for long-term treatment due to the ease of catheter placement. B. Subclavian venous access has the highest rate of infection and should only be used emergently. C. Peripheral venous access is preferred for all procedures when possible. D. Arteriovenous (AV) fistulas should never be accessed for apheresis procedures. E. Carotid artery access is preferred for apheresis procedures because it allows the highest flow rates. 16. A 54-year-old man with a new diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) has been referred to you for TPE. What would be the best course of treatment for this patient? A. Perform TPE 2 to 3 times per week using albumin as replacement until improvement is seen. B. Perform TPE 1 to 2 times per month using albumin as replacement until improvement is seen. C. Perform TPE 2 to 3 times per week using FFP as replacement until improvement is seen. D. Perform TPE 1 to 2 times per month using FFP as replacement until improvement is seen. E. Recommend corticosteroids or intravenous immunoglobulin (IVIg), both of which are more efficacious. 17. TPE is a first-line therapy for which one of the following indications? B. Diarrhea-associated hemolytic uremic syndrome. C. Posttransfusion purpura. D. Cryoglobulinemia. E. Pemphigus vulgaris. 18. You are called to consult on a 33-year-old male patient admitted for hemoptysis and hematuria. Renal biopsy demonstrated crescent formation and linear deposits of IgG along the glomerular basement membrane (GBM). Laboratory testing reveals the presence of anti-GBM antibodies, but anti-neutrophil cytoplasmic antibodies (ANCAs) are not detected. Based on these findings, which one of the following statements is true? A. A combination of TPE, cyclophosphamide, and steroids is the treatment of choice. B. TPE should be reserved for patients who are dialysis dependent. C. Chronic immunosuppression and repeated courses of TPE are required to prevent relapse. D. TPE is ineffective in patients who present with diffuse alveolar hemorrhage. E. Plasma is the only replacement fluid to be used due to the high risk of diffuse alveolar hemorrhage. 19. Which statement regarding hematopoietic progenitor cell (HPC) collection is true? A. The number of CD34+ cells usually decreases during the procedure. B. CD34+ cells are found within the mononuclear cell layer during the procedure. C. CD34+ cell counts peak after 7 days of granulocyte colony-stimulating factor (G-CSF) administration. D. Patients are not at a higher risk of citrate toxicity during large-volume leukapheresis. E. HPCs are not found in the peripheral blood of healthy individuals. 20. A 35-year-old female patient presents to her primary care physician complaining of “foamy” urine and bilateral lower extremity edema. She has a history of fatigue, a malar rash, diffuse myalgias, and arthralgias. Physical exam is remarkable for a blood pressure of 155/100 mm Hg and 3 + bilateral lower extremity pitting edema. Her urinalysis is remarkable for showing a specific gravity of 5.0, blood of 3 +, protein of 4 +, 12 to 16 RBCs per high-power field, rare hyaline casts, and 3 RBCs casts. Which one of the following is the most appropriate course of treatment? A. TPE every other day for five treatments using albumin as replacement. B. Corticosteroid therapy and an angiotensin-converting enzyme inhibitor (ACEI). C. Corticosteroid therapy and TPE every other day for five treatments using albumin as replacement. D. Corticosteroid therapy, ACEI, and TPE every other day for five treatments using albumin as replacement. E. Corticosteroid therapy, cyclophosphamide, ACEI, and TPE every other day for five treatments using albumin as replacement. 21. Which statement is true regarding the use of TPE in the treatment of myasthenia gravis? A. Antibodies against the acetylcholine receptor must be present for TPE to be effective. B. Response to TPE is rapid and symptoms usually improve within 1 to 7 days. C. Immunosuppressive medications should be held until after the course of TPE is completed. D. TPE is less effective than IVIg and should only be used to treat refractory patients. E. Use of TPE prior to thymectomy should be avoided due to the removal of coagulation factors. 22. Which statement about heart transplantation in adults is true? A. Extracorporeal photopheresis (ECP) is used prophylactically to prevent acute cellular rejection. B. Transplantation across ABO types is easily managed with TPE and immunosuppression. C. TPE is a first-line therapy for acute cellular rejection. D. FFP is the replacement fluid of choice during TPE due to the high incidence of coagulopathy. E. Acute antibody-mediated rejection is most commonly seen in this setting. 23. Plerixafor is a mobilization agent used in combination with G-CSF to collect hematopoietic stem cells. Which one of the following best describes the mechanism of action of plerixafor? A. Increased production of HPCs in the bone marrow. B. Decreased destruction of HPCs in the peripheral blood. C. Increased release of HPCs from the bone marrow. D. Increased maturation of HPCs in the bone marrow. E. Increased activation of HPCs in the peripheral blood. 24. For which one of the following renal diseases is TPE considered a first-line treatment? A. Focal segmental glomerulosclerosis. B. Antibody-mediated rejection of a renal allograft. C. Diarrhea-associated hemolytic uremic syndrome. D. Immune complex–induced rapidly progressive glomerulonephritis. E. Myeloma kidney. 25. For which one of the following is TPE indicated in the setting of solid organ transplantation? A. To decrease RBC alloantibody titers. B. To decrease donor-specific anti-HLA titers. C. To decrease donor-specific anti–human platelet antigen (HPA) antibody titers. D. To decrease complement levels. E. To decrease donor-specific anti–human neutrophil antigen (HNA) antibody titers. 26. A 34-year-old woman presents to your hospital with fever and the new onset of mental status changes. She is found to be anemic, with a hemoglobin of 8.2 g/dL (reference range 12.0 to 15.8 g/dL) and thrombocytopenic, with a platelet count of 7 × 109/L (reference range 165 to 415 × 109/L), and with a few schistocytes identified on a peripheral blood smear. Which one of the following statements is the best course of action? A. Transfuse 1 U RBCs and 1 dose of single-donor apheresis platelets. B. Transfuse 10 U cryoprecipitate. C. Transfuse 2 U plasma. D. Initiate a series of plasmapheresis procedures. E. Perform a whole blood exchange. 27. Which statement is true regarding ASFA guidelines for therapeutic apheresis? B. Category II indications include diseases and syndromes for which apheresis is the primary treatment in conjunction with another treatment modality. C. Category III indications include diseases and syndromes for which apheresis is a second-line therapy. D. Category IV indications include diseases and syndromes for which apheresis is the last line of treatment. E. Category V indications include diseases and syndromes for which apheresis is ineffective or harmful. 28. The ASFA periodically publishes guidelines, using an evidence-based approach, regarding the use of therapeutic apheresis in clinical practice. Based on the 2013 ASFA guidelines, there are four categories describing the indications for therapeautic apheresis. According to the 2013 ASFA guidelines, which one of the following is the correct category for severe malaria? B. Disorder for which apheresis is accepted as second-line therapy, either as a primary stand-alone treatment or in conjunction with other modes of treatment. C. The optimal role of apheresis is not established; decision making should be individualized. D. Disorder in which published evidence demonstrates or suggests that apheresis is ineffective or harmful. E. Disorder for which approval for a research study is required from an IRB. 29. The ASFA periodically publishes guidelines, using an evidence-based approach, regarding the use of therapeutic apheresis in clinical practice. Based on the 2013 ASFA guidelines, there are four categories describing the indications for therapeautic apheresis. According to the 2013 ASFA guidelines, which one of the following is the correct category for hematopoietic stem cell transplant-associated thrombotic microangiopathy? B. Disorder for which apheresis is accepted as second-line therapy, either as a primary stand-alone treatment or in conjunction with other modes of treatment. C. The optimal role of apheresis is not established; decision making should be individualized. D. Disorder in which published evidence demonstrates or suggests that apheresis is ineffective or harmful. E. Disorder for which approval for a research study is required from an IRB. 30. In the context of therapeutic plasmapheresis, which one of the following is a characteristic of an “ideal solute”? A. It equilibrates between the intravascular and extravascular spaces. B. It is synthesized during the time frame of the plasmapheresis procedure. C. It is catabolized during the time frame of the plasmapheresis procedure. D. It is completely intravascular. E. It is an IgG antibody. Major points of discussion ■ Peritransplant plasmapheresis in conjunction with immunosuppression and/or IVIg may lower antibody titers and prevent hyperacute rejection of ABO-incompatible solid organ allografts. ■ The replacement fluid for plasmapheresis is albumin, FFP, or a combination of the two, depending on the presence or absence of a coagulopathy. ■ The goal of plasmapheresis is to reduce anti-A and/or anti-B antibody titers to a level low enough to prevent hyperacute rejection. Titers of 4 to 16 have been used for kidney transplantation and titers of 8 to 64 for liver transplantation. ■ The number of peritransplant plasmapheresis procedures depends on the initial isohemagglutinin titer. 2a. A. Plasmapheresis. 2b. A. A Major points of discussion ■ The efficiency of the apheresis procedure decreases as the procedure progresses. ■ Blood component separation can be accomplished by centrifugation or filtration. In a continuous-flow centrifugation device, blood is separated by density and the resulting layers can be removed selectively (e.g., plasma, platelets, leukocytes, RBCs). Filtration devices, which are less commonly used, separate plasma from cells using a microporous filter. ■ Selective absorption using affinity columns exist for removing specific components from plasma, such as LDLs or immunoglobulins. ■ Photopheresis is a procedure in which the WBC layer is collected and treated with 8-methoxypsoralen and ultraviolet light. This crosslinks DNA and induces apoptosis. It was initially developed for treating cutaneous T-cell lymphoma; however, it is currently used in treating organ rejection (heart and lung) and graft-versus-host disease. 3. A. TPE is considered to be first-line therapy. Major points of discussion ■ Each disorder is assigned a category (i.e., I to IV) and strength of recommendation. ■ TPE has been shown to be a second-line therapy, either alone or in conjunction with other immunosuppressive therapy, to desensitize patients with a positive crossmatch to a living donor kidney caused by donor-specific HLA antibodies. ■ Immunosuppressive medications, either alone or in combination with therapeutic plasmapheresis, high-dose IVIg, and/or rituximab, can be used to prevent and treat antibody-mediated rejection. ■ Patients may develop anti-HLA antibodies through prior transfusions, pregnancies, and/or transplantation. ■ Removal of donor-specific HLA antibodies prior to transplantation could enable a negative crossmatch and prevent hyperacute/acute rejection. ■ Posttransplant immunosuppression can prevent loss of the organ caused by an anamnestic response.8 4. A. 2.5 to 5 L. Major points of discussion ■ The total blood volume in adults can be estimated as 70 mL/kg; thus, a 70-kg adult will have a approximately 5-L total blood volume. ■ RBCs should not be transfused in patients with leukostasis until cytoreduction is achieved to prevent further increases in whole blood viscosity. ■ The leukapheresis procedure is expected to lower the WBC count by 30% to 60%. The procedure is not more effective because WBCs are not “ideal solutes.” ■ Studies suggest that prompt treatment of leukostasis with leukapheresis only reduces the risk of early death in this setting. 5. A. No specific therapy is indicated. Major points of discussion ■ In a landmark, multicenter, randomized control study, conservative management consisting of transfusion to a hemoglobin of 10 g/dL was as effective as RCE but associated with fewer transfusion-related complications. ■ The fraction of RBCs remaining refers to how many of the original circulating RBCs remain after the exchange procedure. The lower the fraction, the larger is the volume to be processed and the longer the length of the procedure. A setting of 0.3 is most commonly used as it provides a good balance between the volume of blood required for the procedure and the desired decrease in circulating hemoglobin S levels. ■ In general, sickle cell anemia patients are not transfused to hemoglobin levels above 10.0 g/dL to prevent increased viscosity, which is associated with higher hemoglobin levels in the presence of sickle erythrocytes. ■ Chronic RCEs are useful for preventing severe manifestations of sickle cell anemia, such as stroke and acute chest syndrome.10 6. A. The unit was the first of the four used for the exchange. Major points of discussion ■ Blood transfused into patients with sickle cell anemia should be tested for hemoglobin S trait and only transfused if negative. However, blood is not routinely tested for other hemoglobinopathies and it is not uncommon to observe other variant hemoglobins present in the transfused individuals. ■ “Ideal solutes” are those that are predominantly intravascular and can be removed effectively by the apheresis procedure. A single plasma volume exchange will remove approximately 63% of an ideal solute. A double plasma volume exchange will remove approximately 86% of an ideal solute, and a triple plasma volume exchange will remove 95% of an ideal solute. ■ Examples of molecules or cells that approximate ideal solutes include fibrinogen, IgM antibodies, and RBCs. IgG antibodies and leukemic WBCs are not ideal solutes. ■ The ASFA publishes the guidelines for TPE. The evidence and lack of evidence for these procedures are reviewed periodically by a committee in charge of setting the guidelines. 7. A. Transfuse 2 U RBCs. Major points of discussion ■ Chronic transfusion (simple and/or exchange) therapy is effective in primary and secondary prevention of stroke in patients with sickle cell anemia. ■ The risks of RCE include all the risks of transfusions, such as febrile nonhemolytic transfusion reactions, hemolytic transfusion reactions (delayed/acute), allergic reactions, and transfusion-transmitted infections. ■ RBC units destined for patients with sickle cell anemia should be screened for sickle trait. Thus, patients with sickle cell anemia should receive only sickle-negative blood. ■ The goal of chronic transfusion therapy for sickle cell anemia patients is to maintain the percentage of hemoglobin S at less than 30%. This has been shown to reduce the incidence of stroke and other disease manifestations, such as pain crises and acute chest syndrome.1 8. A. Warm autoimmune hemolytic anemia. Major points of discussion ■ Hyperviscosity causes an increased shear stress in the microvasculature, leading to endothelial damage. ■ Hypervolemia may also occur, leading to respiratory compromise, congestive heart failure, and coma. ■ This is most common in monoclonal gammopathies with IgM paraproteins. ■ There is a logarithmic increase in serum viscosity with increases in the monoclonal protein. ■ TPE is a category I indication to prophylactically decrease paraprotein levels in patients taking rituximab with IgM greater than 5000 mg/dL, or in the treatment of symptomatic hyperviscosity syndrome caused by monoclonal gammopathy. 9. A. To improve the efficacy of chemotherapy by decreasing the overall tumor burden. Major points of discussion ■ Leukocytapheresis should be considered in the treatment of patients with acute myeloid leukemia and a WBC count more than 100 × 109/L and patients with acute lymphoblastic leukemia and a WBC count more than 400 × 109/L. ■ Leukostasis can lead to organ and tissue dysfunction and ischemia resulting from obstruction of the microvasculature. ■ Leukocytapheresis has been shown to improve the pulmonary and neurologic complications seen with leukostasis in acute leukemia. ■ Induction chemotherapy should not be delayed for leukocytapheresis to be completed. ■ From 1.5 to 2 total blood volumes should be processed. ■ Reductions in the WBC count and blast count cannot be predicted reliably because additional marginated leukemic cells may be released into the circulation and leukemic blasts may not be as efficiently removed by centrifugation as expected. 10. A. RCE can be used in conjunction with antimalarial therapy to treat uncomplicated malaria when available. Major points of discussion ■ Severe cases may be complicated by severe anemia, pulmonary edema, seizures, shock, disseminated intravascular coagulopathy, renal failure, metabolic derangements, coma, and death. ■ Antimalarial therapy should not be postponed but should be started immediately. ■ Most severe cases are due to Plasmodium falciparum. ■ From 1 to 2 RBC volumes should be processed using donor RBCs as the replacement fluid. ■ Complications of RCE for malaria include any complication associated with RBC transfusion and apheresis procedures. ■ These complications include transfusion reactions, RBC alloimmunization, hypocalcemia, hypovolemia, bleeding, and infection. 11. A. A 65-year-old man with essential thrombocythemia and a history of myocardial infarction with a platelet count of 400 × 109/L. Major points of discussion ■ Thrombocytosis may be primary or secondary. ■ Primary thrombocytosis is seen in myeloproliferative disorders, including essential thrombocythemia and chronic myelogenous leukemia. Complications of thrombocytosis, such as thrombosis and hemorrhage, may be seen in these patients. ■ Risk factors for thrombosis and hemorrhage in these patients include patient age older than 60 years, a history of cardiovascular disease, and a history of thrombosis. ■ Secondary thrombocytosis can be associated with multiple disorders, including malignancy, splenectomy, infection, and chronic inflammation. Complications of thrombocytosis are rarely seen in these patients. ■ Medical management to decrease the platelet count using hydroxyurea, anagrelide, or interferon alfa should be considered prior to therapeutic plateletpheresis. Plateletpheresis is usually used only in rare cases. ■ From 1.5 to 2 total blood volumes are usually processed in one procedure; the decrease in platelet count cannot be reliably predicted based on the processed blood volume. Treatment should be guided by intraprocedure platelet counts.8 12. A. Disorders for which apheresis is accepted as first-line therapy, either as a primary stand-alone treatment or in conjunction with other modes of treatment. Major points of discussion ■ In the 2013 ASFA guidelines, the descriptions of the various categories were simplified compared with previous versions of the guidelines. Changes in these descriptions include the addition of the strength of the recommendation, which allowed categorization to be better aligned with the strength of the evidence and the quality of the relevant publications in the literature. ■ The definition of category III reflects the individual character of the decision-making process for diseases in this category. ■ The recommendation grade and the individual patient’s clinical circumstances should guide whether or not therapeutic apheresis is included in the treatment plan for category III indications. ■ Other professional organizations in other fields (e.g., neurology) also prepare consensus guidelines regarding the indications for therapeutic apheresis. These may not always agree with those promulgated by the ASFA.8 13. A. TPE has an immunomodulatory effect by decreasing ADAMTS13 autoantibody production by plasma cells. Major points of discussion ■ In practice, thrombocytopenia and MAHA (noted as anemia with schistocytes or fragmented RBCs on peripheral blood smear and an elevation in lactate dehydrogenase) without other clinical explanations (i.e., disseminated intravascular coagulation) are sufficient to diagnose TTP and initiate treatment. ■ TPE should be started as soon as possible due to the high mortality rate associated with TTP. ■ TTP is associated with a severe deficiency in ADAMTS13 (i.e., adisintergrin and metalloproteinase with a thrombospondin type 1 motif, member 13) enzyme activity, which cleaves ultra-large von Willebrand multimers. ■ The majority of idiopathic cases of TTP are due to an autoantibody against ADAMTS13. ■ Congenital TTP (i.e., Upshaw-Schulman syndrome) is associated with somatic mutations in the ADAMTS13 gene that lead to a severe decrease in ADAMTS13 activity. ■ TPE is thought to remove the autoantibody and replace ADAMTS13 protease activity, which is found in FFP. ■ FFP or cryoprecipitate-reduced plasma should be used as the replacement fluid to provide a source of ADAMTS13. 14. A. Renal transplantation with antibody-mediated rejection. Major points of discussion ■ Five percent albumin, with or without normal saline, is the most commonly used replacement fluid. ■ Five percent albumin will maintain normal oncotic pressure with a minimal risk of allergic reaction and infectious disease transmission. ■ FFP is used as a replacement fluid in patients with TTP, coagulation factor deficiencies, or other bleeding risks. ■ FFP is associated with an increased risk of allergic reactions, infectious disease transmission, other transfusion reactions, and citrate toxicity due to anticoagulation with citrate. ■ In TTP, FFP or cryoprecipitate-reduced plasma is used to replace ADAMTS13 protease activity. ■ Patients who undergo daily plasmapheresis with albumin replacement may become deficient in fibrinogen (most common) or other coagulation factors; in this setting, FFP may be used in conjunction with albumin as a replacement fluid. ■ RBCs are used as a replacement product in RCE procedures. 15. A. Femoral venous access is preferred for long-term treatment due to the ease of catheter placement. Major points of discussion ■ Peripheral veins are the first choice for venous access due to the low risk of adverse events. However, most patients’ peripheral veins are not adequate to withstand multiple repetitive apheresis procedures. ■ Central venous catheters must have a double lumen to allow for blood draw and return. They must be staggered (proximal and distal port) to prevent mixing of returned and drawn blood. They also must be firm enough to prevent collapse due to high pressure. ■ Central venous access may be complicated by infection, hemorrhage, thrombosis, pneumothorax, and arrhythmias. ■ Femoral venous access • Advantages: Ease of insertion, may be placed at the bedside, easier to control hemorrhage • Disadvantages: Increased risk of infection, risk of femoral artery puncture, increased risk of thrombosis, limited patient mobility ■ Subclavian/internal jugular access • Advantages: Long-term placement, lower risk of infection • Disadvantages: Hemothorax, pneumothorax, cardiac arrhythmias, more difficult to place, requires radiographic confirmation of correct placement 16. A. Perform TPE 2 to 3 times per week using albumin as replacement until improvement is seen. Major points of discussion ■ It is thought to be due to a B-cell and T-cell autoimmune process, possibly mediated by autoantibodies recognizing myelin-associated glycolipid antigens, leading to demyelination of peripheral nerves. ■ CIDP is a category I indication for TPE according to the ASFA guidelines published in 2013. ■ From 1 to 1.5 total plasma volumes are removed using albumin, with or without saline, as the replacement fluid. ■ TPE is usually performed 2 to 3 times per week until symptom improvement is noted. Treatment may then be tapered. ■ Relapse may be seen with discontinuation of treatment and maintenance therapy is often necessary.8 17. A. Rheumatoid arthritis. Major points of discussion ■ Cryoglobulins are immunoglobulins that precipitate at low temperatures. ■ There are three types of cryoglobulins: 2. Type II cryoglobulins are monoclonal proteins, usually IgM, with a polyclonal component, usually IgG. These “mixed” cryoglobulins are usually associated with hepatitis C. 3. Type III cryoglobulins are polyclonal immunoglobulins, usually IgG or IgM. Commonly, a polyclonal anti-IgG IgM forming precipitating immune complexes is seen. These are usually associated with hepatitis C, autoimmune diseases, and inflammatory conditions. ■ The immunoglobulins and immune complexes precipitate in areas of the body exposed to lower temperatures, thereby causing small-vessel damage. ■ TPE can be used to decrease cryoglobulin levels. ■ TPE is used most often to treat severe cryoglobulinemia in patients with renal impairment, vasculitis, and/or neuropathy. ■ Severe, symptomatic cryoglobulinemia is a category I indication for TPE according to the ASFA guidelines published in 2013. ■ Cryoglobulinemia secondary to hepatitis C is a category II indication for TPE according to the ASFA guidelines published in 2013.2,8 18. A. A combination of TPE, cyclophosphamide, and steroids is the treatment of choice. Major points of discussion ■ RPGN is separated into three categories based on immunofluorescence staining on renal biopsy. The clinical presentation, response to treatment, and prognosis differ among the three categories. ■ The three categories are anti-GBM antibody disease (Goodpasture disease), immune complex disease, and pauci-immune disease. ■ Anti-GBM disease is characterized by linear deposits of IgG along the GBM and is caused by an autoantibody to collagen type IV. ■ From 10% to 40% of affected patients may have detectable ANCA. ■ Clinically, these patients may present with renal manifestations, including hematuria, oliguria, and anuria, and pulmonary manifestations, including cough, hemoptysis, and diffuse alveolar hemorrhage. ■ Without treatment, anti-GBM disease is rapidly progressive with a high mortality rate. A combination of TPE, cyclophosphamide, and steroids is the treatment of choice and should be started as early as possible. ■ Anti-GBM disease that is dialysis independent or accompanied by diffuse alveolar hemorrhage is a category I indication for TPE based on the ASFA guidelines published in 2013. ■ The renal dysfunction in patients with anti-GBM disease who are dialysis dependent is unresponsive to TPE and the renal damage is irreversible. This is a category IV indication for TPE. 19. A. The number of CD34+ cells usually decreases during the procedure. Major points of discussion ■ HPCs are present in both the bone marrow and the peripheral blood in adults. ■ Peripheral blood–derived HPCs are easier to collect and lead to more rapid engraftment compared with bone marrow–derived HPCs. ■ Administration of hematopoietic cytokines, such as G-CSF, is required to cause a transient increase of circulating HPCs in the peripheral blood. ■ Large-volume leukapheresis (LVL) is usually performed to collect adequate numbers of CD34+ cells in the fewest number of procedures possible. ■ From 15 to 40 L of blood may be processed during LVL. ■ Patients undergoing LVL are at increased risk of citrate toxicity and thrombocytopenia. 20. A. TPE every other day for five treatments using albumin as replacement. Major points of discussion ■ Lupus nephritis is a category IV indication for TPE according to the ASFA 2013 guidelines. ■ TPE has not been shown to demonstrate any benefit in treating lupus nephritis. ■ Corticosteroid therapy is used in patients with clinically significant renal disease. Cyclophosphamide or other immunosuppressive agents may be used in severe cases or in patients who are unable to tolerate corticosteroid therapy. ■ Hypertension should be treated aggressively in these patients. ■ Severe systemic lupus erythematosus is a category II indication for TPE according to the ASFA 2013 guidelines.8 21. A. Antibodies against the acetylcholine receptor must be present for TPE to be effective. Major points of discussion ■ Anti-AChR antibodies are detectable in 80% to 90% of patients. These antibodies can block impulses sent by afferent nerves, decrease the number of available receptors at the motor endplate, or lead to cellular damage. ■ Antibodies against the muscle-specific receptor tyrosine kinase (anti-MusK) are found in approximately 50% of patients without identifiable anti-AChR antibodies. ■ Myasthenia gravis may be treated with cholinesterase inhibitors, immunosuppressive medications, TPE, and IVIg. ■ There is an increased incidence of thymomas in patients with myasthenia gravis. Thymectomy can produce disease remission or improvement in patients both with and without thymomas. ■ Myasthenia gravis (moderate and severe) and pre-thymectomy are category I indications for using TPE according to the ASFA guidelines published in 2013. ■ TPE should consist of 1 to 1.5 total plasma volumes using albumin, with or without saline, as replacement fluid.8 22. A. Extracorporeal photopheresis (ECP) is used prophylactically to prevent acute cellular rejection. Major points of discussion ■ ABO or major HLA incompatibility can lead to hyperacute rejection and cardiac allograft failure. ■ ABO-incompatible heart transplantation in adults is avoided but may be performed in children up to 40 months of age. ■ ABO-incompatible heart transplantation is more successful in young children and infants due to lower anti-A and anti-B antibody titers and the relative immaturity of their immune systems. ■ ABO antigens are expressed on endothelial cells. ■ ECP may be used to prevent (category I indication) or treat (category II indication) acute cellular rejection in heart transplantation. ■ ECP uses ultraviolet light in the presence of a psoralen to induce DNA damage, leading to immunomodulation, destruction of allograft-specific T-cell populations, and induction and expansion of regulatory T cells. The exact mechanism by which ECP affects the immune system is currently unknown. ■ TPE may be used to treat acute antibody-mediated cardiac allograft rejection (category III indication), but the efficacy is not conclusively documented at this time. 23. A. Increased production of HPCs in the bone marrow. Major points of discussion ■ HPCs are present in the bone marrow and peripheral blood in adults. ■ CD34+ HPCs are present in very small quantities (< 1%) in the peripheral blood. ■ Administration of hematopoietic cytokines causes transient increases of HPCs in the peripheral blood. ■ Plerixafor is a reversible inhibitor of stromal cell–derived factor-1 binding to its chemokine receptor (i.e., CXCR-4). ■ Binding of stromal cell–derived factor-1 to CXCR-4, along with interactions with other adhesion molecules, leads to trafficking and adhesion of HPCs to the stem cell niche within the bone marrow. ■ Plerixafor is administered after four daily doses of G-CSF, 10 to 11 hours prior to the start of collection, and every subsequent day with G-CSF for a maximum of four doses after the start of collection. ■ Peripheral blood–derived HPCs are easier to collect and lead to more rapid engraftment compared with bone marrow–derived HPCs. ■ Plerixafor is FDA approved for use in combination with G-CSF for the mobilization of autologous HPCs for peripheral blood collection and subsequent autologous transplantation in patients with multiple myeloma or non-Hodgkin lymphoma.9 24. A. Focal segmental glomerulosclerosis. Major points of discussion ■ Antibody-mediated rejection of renal allografts is due to either preformed or de novo donor-specific antibodies. ■ Patients with antibody-mediated renal allograft rejection should start immunosuppressive therapy to limit antibody production prior to starting TPE.8 25. A. To decrease RBC alloantibody titers. Major points of discussion ■ Peritransplant TPE in conjunction with immunosuppression and/or IVIg may lower antibody titers and prevent hyperacute rejection. ■ The replacement fluid for plasma exchange is albumin, FFP, or a combination of the two, depending on the presence or absence of coagulopathy. ■ The goal of TPE in this setting is to reduce antibody titers to a low enough level to prevent hyperacute rejection. Acceptable titers for transplantation depend on the type of organ being transplanted and type of antibodies present. ■ The number of peritransplant plasma exchange procedures depends on the initial antibody titers. ■ The use of TPE to treat antibody-mediated rejection in the setting of renal transplantation is a category I indication based on ASFA guidelines published in 2013.8 26. A. Transfuse 1 U RBCs and 1 dose of single-donor apheresis platelets. Major points of discussion ■ TTP is a characterized by a pentad of clinical findings: thrombocytopenia, MAHA, mental status changes, renal failure, and fever. ■ This patient most likely has TTP based on her presenting signs and symptoms: fever, thrombocytopenia, MAHA, and mental status changes. ■ The presence of these findings in the absence of another clinical explanation is sufficient to start plasmapheresis emergently due to the high mortality of untreated TTP. ■ If plasmapheresis is not available, plasma should be transfused to replace ADAMSTS13, the metalloproteinase that cleaves ultra-large von Willebrand multimers, which is deficient in TTP patients. ■ Plasmapheresis should be performed daily until the platelet count is greater than 150 × 109/L and lactate dehydrogenase (LDH) levels are close to normal values for 2-3 consecutive days.6,8 27. A. Category I indications include diseases and syndromes for which apheresis is the primary treatment. Major points of discussion ■ The ASFA issues guidelines are based on current evidence for the use of therapeutic apheresis. ■ Four categories are used to define the indications for apheresis for diseases and syndromes. ■ The strength of the recommendation for the use of apheresis is described, as well as based on the quality of the evidence that is currently available. ■ Category I indications include disorders for which apheresis is a first-line therapy, either alone or in conjunction with other treatment modalities (e.g., TTP). ■ Category II indications include disorders for which apheresis is a second-line therapy, either alone or in conjunction with other treatment modalities (example: ABO-incompatible hematopoietic cell transplant). ■ Category III indications include disorders for which the efficacy and role of apheresis has not been established (example: sepsis with multiorgan failure). ■ Category IV indications include disorders for which apheresis is ineffective or harmful (e.g., schizophrenia).8 28. A. Disorder for which apheresis is accepted as first-line therapy, either as a primary stand-alone treatment or in conjunction with other modes of treatment. Major points of discussion ■ In the 2013 ASFA guidelines, the descriptions of the various categories were amended and simplified in comparison to previous editions of the guidelines. Changes in the descriptions of the categories included the addition of the strength of the recommendation, which allowed categorization to be better aligned with the strength of the evidence and the quality of the relevant publications in the literature. ■ The current description of category II is disorders for which apheresis is accepted as second-line therapy, either as a stand-alone treatment or in conjunction with other modes of treatment. For severe malaria, the recommendation grade is 2B, which is a weak recommendation supported only by moderate quality evidence, such as controlled trials. ■ RCE (automatic or manual) in severely ill patients with high levels of parasitemia (i.e., > 10%) is believed to improve the rheological properties of the blood and to reduce levels of parasite-derived toxins, hemolytic metabolites, and cytokines. It is an adjunct therapy to the use of specific anti-malarial agents.3,5,8 29. A. Disorder for which apheresis is accepted as first-line therapy, either as a primary stand-alone treatment or in conjunction with other modes of treatment. Major points of discussion ■ In the 2013 ASFA guidelines, the descriptions of the various categories were amended and simplified compared with the previous editions of the guidelines. Changes in the descriptions of the categories included the addition of the strength of the recommendation, which allowed categorization to be better aligned with the strength of the evidence and the quality of the relevant publications in the literature. ■ The current definition of category III reflects the individual character of the decision-making process for diseases in this category. The recommendation grade and individual patient’s clinical circumstances should guide inclusion of therapeutic apheresis in the treatment plan for category III indications. The current description of category III is as follows: Disorders in which the optimum role of apheresis is not established and decision making should be individualized. For transplantation-associated thrombotic microangiopathy, the recommendation grade is 2C. ■ Although a consensus statement by the Bone Marrow and Transplant Clinical Trials Network Toxicity Committee recommends that therapeutic plasmapheresis not be considered as a standard of care for this entity, some patients do respond and treatment decisions should be individualized for patients with persistent transplantation-associated thrombotic microangiopathy.4,7,8 30. A. It equilibrates between the intravascular and extravascular spaces. Major points of discussion ■ The synthetic rate, fractional catabolic rate, and intercompartmental movement of an ideal solute are balanced in a steady state and proceed much slower than the actual removal of plasma from the intravascular space. ■ IgM is 76% intravascular, whereas IgG is only 45% intravascular. Thus, plasmapheresis is much more efficient at removing IgM antibodies. ■ Fibrinogen and IgM approximate the characteristics of a soluble ideal solute; IgG does not behave as an ideal solute. For cytapheresis (e.g., erythrocytaphereis, leukapheresis), RBCs, which travel in the laminar flow, approximate the characteristics of a cellular “ideal solute,” whereas leukocytes, which can marginate, do not behave as ideal solutes. ■ After a 1–plasma volume exchange, approximately 37% of an ideal solute will still remain in the circulation.11
Transfusion Medicine
Therapeutic Apheresis
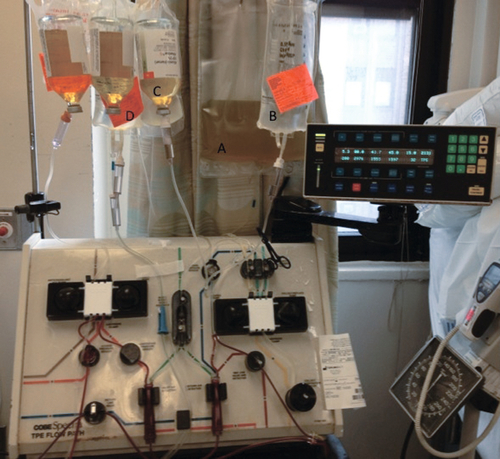
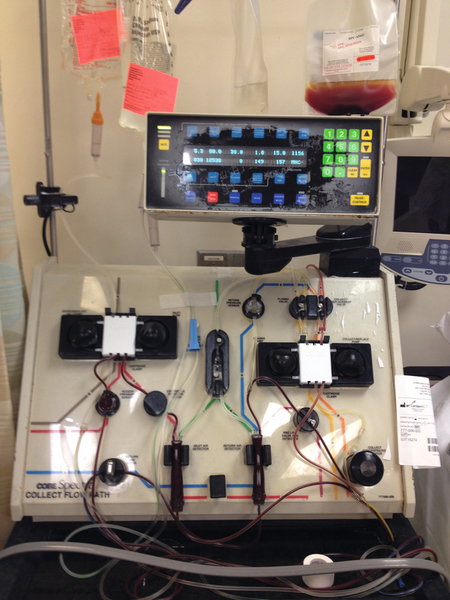
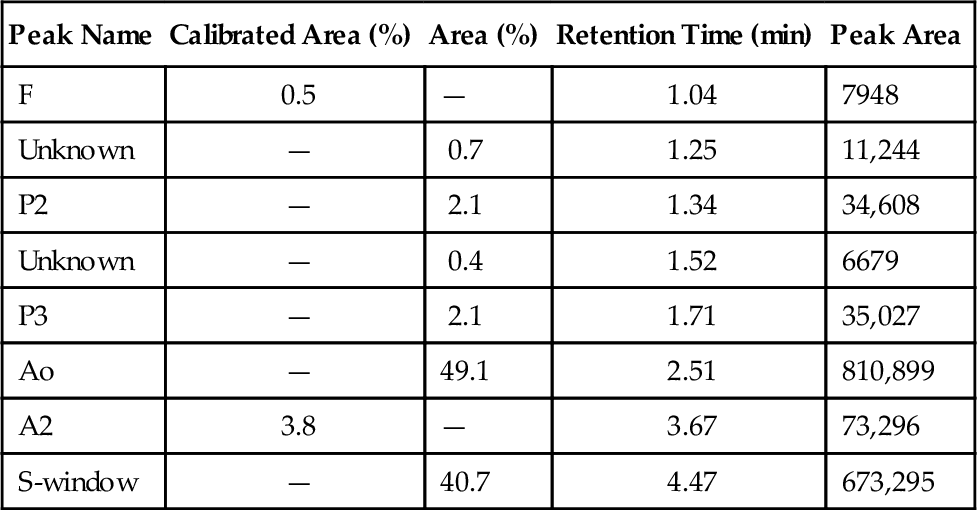
Rationale: The picture represents a standard setup for a plasmapheresis procedure using albumin and saline for replacement fluids.
B. Plateletpheresis.
Rationale: Hints that this is not a plateletpheresis include the absence of platelets in the collection bag and the use of multiple albumin bottles for replacement fluid, which would be unnecessary for a plateletpheresis procedure.
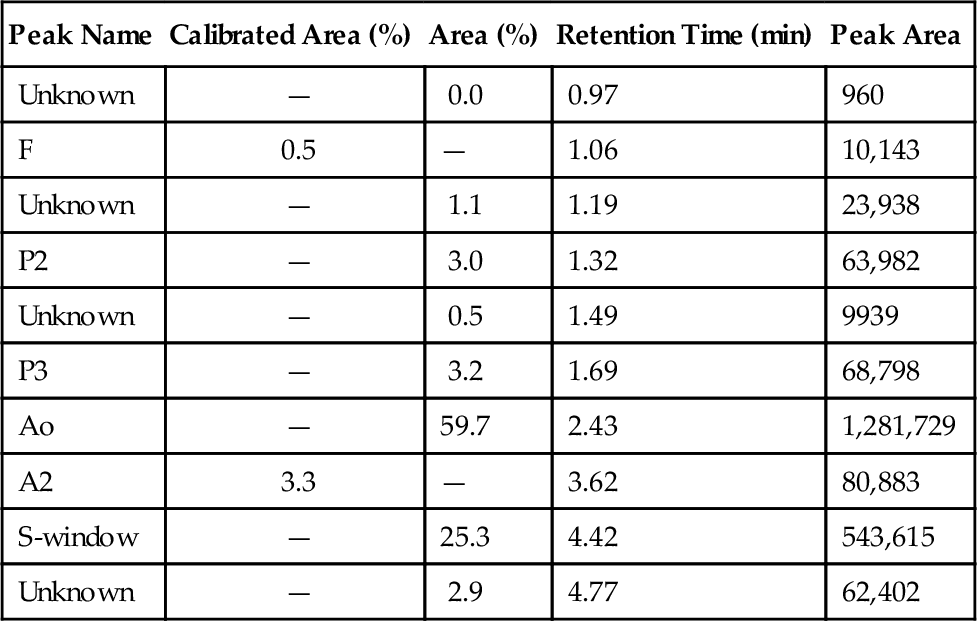
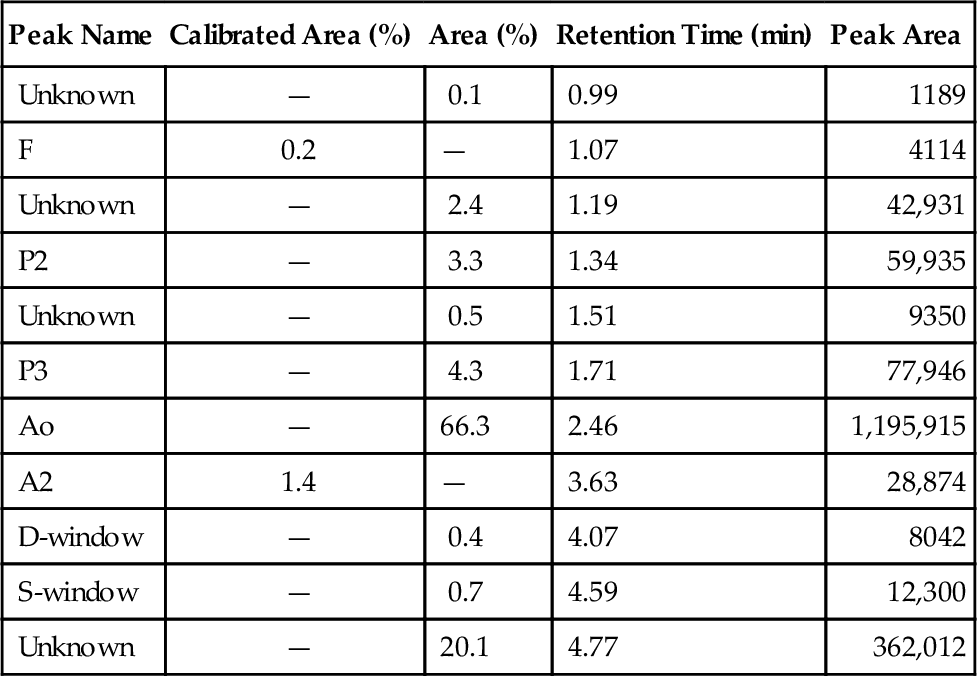
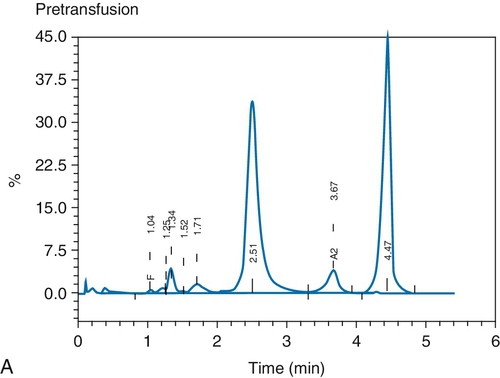
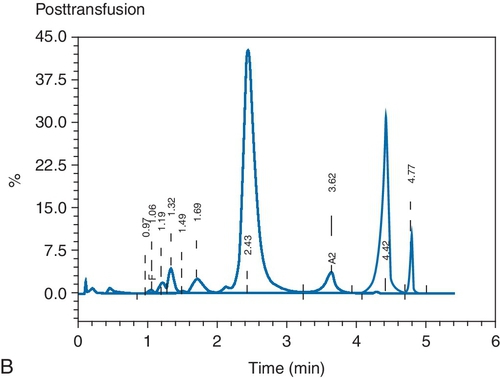
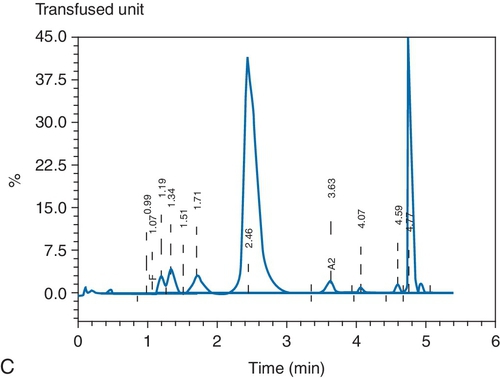
C. Red blood cell (RBC) exchange.
Rationale: The collection bag would be filled with RBCs in an RBC exchange.
D. Leukapheresis.
Rationale: Hints that this is not a leukapheresis include the absence of WBCs in the collection bag and the use of multiple albumin bottles for replacement fluid, which would be unnecessary for a leukapheresis procedure.
E. Stem cell collection.
Rationale: Hints that this is not a stem cell collection include the absence of cells in the collection bag and the use of multiple albumin bottles for replacement fluid, which would be unnecessary for a stem cell collection procedure.
Rationale: This is the collection bag, which is expected to contain roughly the amount of fibrinogen as in plasma.
B. B
Rationale: This is the saline replacement bag containing normal saline.
C. C
Rationale: This is a bottle of albumin being used as replacement fluid.
D. D
Rationale: This is a bag of citrate, which is used for anticoagulation during the procedure. Although the citrate label is difficult to see in this figure, the only container expected to have appreciable fibrinogen in a plasmapheresis procedure is the collection bag, unless FFP is used as replacement fluid.
E. None of the labeled containers contains measurable amounts of fibrinogen.
Rationale: The collection bag in a plasmapheresis procedure contains plasma, which contains fibrinogen as an “ideal solute.”
B. TPE is considered to be second-line therapy.
C. The benefit of TPE is unclear.
D. TPE is ineffective.
E. TPE has never been tried.
Rationale: TPE for the desensitization of renal transplant candidates with a positive living donor kidney crossmatch caused by donor-specific HLA antibodies is considered to be second-line therapy, either as a standard treatment or in conjunction with other modes of treatment.
B. 5 to 7.5 L.
C. 7.5 to 10 L.
D. 10 to 15 L.
E. 15 to 20 L.
Rationale: 1.5 to 2 total blood volumes should be processed in a leukapheresis procedure.
Rationale: There are increased complications for sickle cell patients undergoing surgery; thus, some form of therapy to reduce the hemoglobin S percentage is warranted.
B. Begin hydroxyurea and delay surgery until the maximum tolerated dose (MTD) is achieved.
Rationale: Although hydroxyurea is useful for increasing hemoglobin F levels and reducing sickle cell anemia–related complications, this surgery does not have to be delayed until hydroxyurea takes effect.
C. Transfuse 4 U RBCs.
Rationale: This approach will bring the patient’s hemoglobin S level to approximately 50%. Evidence suggests that conservative management raising hemoglobin to 10 g/dL is as effective as exchange transfusions, with fewer side effects.
D. Perform an exchange transfusion, setting the end hemoglobin to 10.0 g/dL and the fraction of cells remaining to 0.3.
Rationale: Although this is an appropriate choice, and some physicians will opt for an exchange transfusion prior to surgery, the evidence suggests that conservative management is as effective with fewer side effects.
E. Perform an exchange transfusion, setting the end hemoglobin to 10.0 g/dL and the fraction of cells remaining to 0.6.
Rationale: Simple transfusion would be as effective in lowering the hemoglobin S level and is associated with less risk to the patient.
B. The unit was the second of the four used for the exchange.
C. The unit was the third of the four used for the exchange.
D. The unit was the fourth of the four used for the exchange.
E. The order of use of the units would not affect the postprocedure percentage of circulating hemoglobin Hasharon.
Rationale: The apheresis procedure is a continuous exchange procedure; thus, RBCs exchanged in the beginning of the procedure are removed as the procedure progresses.
Rationale: This would increase the patient’s hemoglobin level to more than 10 g/dL, which is associated with increased viscosity.
B. Hydroxyurea 20 mg/kg.
Rationale: Although hydroxyurea is useful for increasing hemoglobin F and reducing sickle cell anemia–related complications, this is inappropriate for the treatment of acute chest syndrome.
C. Manual exchange using 6 U RBCs.
Rationale: If an automated exchange were not available, a manual exchange would be an appropriate option.
D. Automated RBC exchange (RCE) transfusion, setting the end hematocrit to 30% and the fraction of RBCs remaining to 0.3.
Rationale: The appropriate settings for an automated exchange would result in a hematocrit of no greater than 30% (i.e., a hemoglobin no greater than 10 g/dL) and hemoglobin S level less than 30%.
E. Partial automated RCE transfusion, setting the end hemoglobin to 12.0 g/dL and the fraction of cells remaining to 0.6.
Rationale: A partial exchange would not reduce the hemoglobin S to less than 30%. Furthermore, the total hemoglobin should not be raised above 10 g/dL.
Rationale: This is a category III indication for TPE. Prednisone is the treatment of choice.
B. Hyperviscosity with monoclonal gammopathy.
Rationale: This is a category I indication for TPE.
C. Bleeding with coagulation factor inhibitors.
Rationale: This is a category III indication for TPE.
D. Thrombosis with antiphospholipid antibody syndrome.
Rationale: This is a category II indication for TPE. TPE may be used in combination with anticoagulants, corticosteroids, and/or IGIg.
E. Bleeding with posttransfusion purpura.
Rationale: This is a category III indication for TPE. High-dose IVIg is the treatment of choice.
Rationale: This is not an indication for the use of leukocytapheresis.
B. To improve long-term mortality in patients with acute myeloid leukemia.
Rationale: Leukocytapheresis has not been shown to improve long-term mortality in acute myeloid leukemia.
C. To prevent leukostasis and the resultant complications in patients with chronic myelogenous leukemia.
Rationale: Currently, leukocytapheresis is not recommended for the treatment of chronic myelogenous leukemia.
D. To treat complications associated with leukostasis in patients with acute myeloid leukemia.
Rationale: Leukocytapheresis is a category I recommendation for the treatment of leukostasis in patients with acute myeloid leukemia.
E. To prevent tumor lysis syndrome in patients with acute lymphoblastic leukemia.
Rationale: Leukocytapheresis has not been shown to be more effective than chemotherapy and supportive care in these patients and is considered a category III indication for prophylaxis for hyperleukocytosis.
Rationale: RCE is not indicated in the treatment of uncomplicated malaria.
B. Antimalarial therapy should be held until after the RCE is completed.
Rationale: Antimalarial therapy is the primary treatment and should be started immediately.
C. There is a high risk of RBC antigen alloimmunization in these patients and RCE should be avoided.
Rationale: These patients are not at any increased risk for RBC alloimmunization.
D. A minimum of two RCEs should be performed to decrease the parasitemia significantly.
Rationale: One or two treatments may be performed.
E. RCE is used only for severe malaria in conjunction with antimalarial therapy.
Rationale: RCE may be used to treat severe malaria (i.e., parasitemia > 10%, renal or pulmonary complications, or cerebral involvement).
Rationale: This patient has a normal platelet count (reference range 165 to 415 × 109/L) and therapeutic plateletpheresis is not indicated.
B. A 20-year-old man status post-splenectomy for a traumatic splenic laceration with a platelet count of 1000 × 109/L.
C. A 45-year-old woman with ovarian carcinoma, a history of thromboembolism, and a platelet count of 750 × 109/L.
Rationale: The efficacy of therapeutic plateletpheresis in the treatment of secondary thrombocytosis has not been proven and has been given a category III recommendation. Medical management with hydroxyurea should be considered first.
D. A 70-year-old man with chronic myelogenous leukemia, a history of myocardial infarction, and a platelet count of 750 × 109/L.
Rationale: Therapeutic plateletpheresis is not a first-line therapy for this patient. A trial of hydroxyurea should be considered first to lower this patient’s platelet count.
E. A 57-year-old woman with chronic myelogenous leukemia and acute hemorrhage, and a platelet count of 750 × 109/L.
Rationale: This patient is the best candidate for therapeutic plateletpheresis due to her acute hemorrhage. Symptomatic thrombocytosis is a category II indication for therapeutic plateletpheresis.
Rationale: This is the description of a category I indication.
B. Disorders for which apheresis is accepted as second-line therapy, either as a stand-alone treatment or in conjunction with other modes of treatment.
Rationale: This is the description of a category II indication.
C. The optimal role of apheresis is not established; decision making should be individualized.
Rationale: This is the description of a category III indication.
D. Disorders in which published evidence demonstrates or suggests that apheresis is ineffective or harmful. Institutional review board (IRB) approval is desirable if apheresis treatment is undertaken in these circumstances.
Rationale: This is the description of category IV indication.
E. Disorders that, based on the available evidence, should never be treated with apheresis.
Rationale: There is no such category described in the ASFA guidelines.
Rationale: TPE has not been shown to have an effect on antibody production by plasma cells in this setting.
B. TPE removes B cells from the circulation, thereby decreasing ADAMTS13 autoantibody production.
Rationale: TPE does not remove significant numbers of B cells from the circulation.
C. TPE removes ADAMTS13 autoantibody and replaces ADAMTS13 protease activity.
Rationale: Based on the available evidence, this is the current rationale for the efficacy of TPE in the treatment of TTP.
D. TPE removes ADAMTS13 protease activity and replaces platelets.
Rationale: TPE does not replace platelets.
E. TPE provides ultra-large von Willebrand multimers to the patient.
Rationale: TPE does not provide ultra-large von Willebrand multimers to the patient.
B. Acute chest syndrome in sickle cell disease.
Rationale: Sickle hemoglobin-negative RBCs should be used to replace the patient’s sickle RBCs.
C. Acute inflammatory demyelinating polyradiculopathy (i.e., Guillain-Barré syndrome).
D. Phytanic acid storage disease (i.e., Refsum disease).
Rationale for A, C, and D: Albumin is the appropriate replacement fluid in these cases.
E. TTP.
Rationale: FFP or cryoprecipitate-reduced plasma is the appropriate replacement fluid in this case.
Rationale: Femoral access is typically only used as a temporary site because it limits patient mobility and can be complicated by infection.
B. Subclavian venous access has the highest rate of infection and should only be used emergently.
Rationale: Femoral venous access is complicated more often by infection than subclavian venous access.
C. Peripheral venous access is preferred for all procedures when possible.
Rationale: The catheter is the greatest risk factor for adverse events during a trial of apheresis and should be avoided when possible.
D. Arteriovenous (AV) fistulas should never be accessed for apheresis procedures.
Rationale: AV fistulas may be used for apheresis procedures as long as the nursing staff members are adequately trained in their use.
E. Carotid artery access is preferred for apheresis procedures because it allows the highest flow rates.
Rationale: The carotid artery is never accessed for therapeutic apheresis procedures.
Rationale: This is the recommended course of TPE for the treatment of CIDP.
B. Perform TPE 1 to 2 times per month using albumin as replacement until improvement is seen.
Rationale: TPE should be performed more often initially until improvement is noted. Then treatment can be tapered.
C. Perform TPE 2 to 3 times per week using FFP as replacement until improvement is seen.
D. Perform TPE 1 to 2 times per month using FFP as replacement until improvement is seen.
Rationale: FFP is not an appropriate replacement fluid for this patient. Additionally, TPE should be performed more often initially until improvement is noted. Then treatment can be tapered.
E. Recommend corticosteroids or intravenous immunoglobulin (IVIg), both of which are more efficacious.
Rationale: TPE, corticosteroids, and IVIg have been shown to be equally effective for the treatment of CIDP.
Rationale: TPE is not recommended for the treatment of rheumatoid arthritis.
B. Diarrhea-associated hemolytic uremic syndrome.
Rationale: TPE is not recommended for the treatment of diarrhea-associated hemolytic uremic syndrome and is a category IV indication.
C. Posttransfusion purpura.
Rationale: TPE is indicated for the treatment of posttransfusion purpura only in patients who are refractory to IVIg treatment and have persistent profound thrombocytopenia. This is a category III indication for TPE.
D. Cryoglobulinemia.
Rationale: TPE is a first-line treatment for cryoglobulinemia.
E. Pemphigus vulgaris.
Rationale: TPE is not recommended for the treatment of pemphigus vulgaris and is a category IV indication.
Rationale: See Major Points of Discussion.
B. TPE should be reserved for patients who are dialysis dependent.
Rationale: TPE is not effective for renal dysfunction once patients are dialysis dependent and should be used only if diffuse alveolar hemorrhage is present.
C. Chronic immunosuppression and repeated courses of TPE are required to prevent relapse.
Rationale: Relapses are not typically seen in patients who are ANCA negative, and treatment can be discontinued once remission is achieved.
D. TPE is ineffective in patients who present with diffuse alveolar hemorrhage.
Rationale: Approximately 90% of patients with diffuse alveolar hemorrhage respond to TPE and it is a category I indication for TPE.8
E. Plasma is the only replacement fluid to be used due to the high risk of diffuse alveolar hemorrhage.
Rationale: Albumin may be used in patients without evidence of coagulopathy or diffuse alveolar hemorrhage. If diffuse alveolar hemorrhage is present, plasma may be used as replacement at the end of the procedure to maintain levels of coagulation factors, particularly fibrinogen.
Rationale: In most cases, the number of CD34+ cells increases during the procedure, thereby allowing more CD34+ cells to be collected than appears based on calculations using preprocedure CD34+ cell counts. The marrow is thought to release additional HPCs into the circulation during the time frame of the procedure.
B. CD34+ cells are found within the mononuclear cell layer during the procedure.
Rationale: A leukapheresis-type procedure is performed, focusing on collecting mononuclear cells.
C. CD34+ cell counts peak after 7 days of granulocyte colony-stimulating factor (G-CSF) administration.
Rationale: CD34+ cell counts usually peak on day 5 after 4 days of G-CSF mobilization. WBC counts will continue to rise.
D. Patients are not at a higher risk of citrate toxicity during large-volume leukapheresis.
Rationale: The increased time and volume of blood processed increases the risk of citrate toxicity.
E. HPCs are not found in the peripheral blood of healthy individuals.
Rationale: HPCs are found in low numbers in the peripheral blood (< 1%).
B. Corticosteroid therapy and an angiotensin-converting enzyme inhibitor (ACEI).
Rationale: This is appropriate therapy for this patient.
C. Corticosteroid therapy and TPE every other day for five treatments using albumin as replacement.
D. Corticosteroid therapy, ACEI, and TPE every other day for five treatments using albumin as replacement.
E. Corticosteroid therapy, cyclophosphamide, ACEI, and TPE every other day for five treatments using albumin as replacement.
Rationale for A, C, D, and E: This patient has systemic lupus erythematosus and currently presents with lupus nephritis. These are not appropriate therapies for this patient.
Rationale: TPE is effective for both seropositive and seronegative patients.
B. Response to TPE is rapid and symptoms usually improve within 1 to 7 days.
Rationale: See Major Points of Discussion.
C. Immunosuppressive medications should be held until after the course of TPE is completed.
Rationale: Immunosuppressive medications should be initiated during the course of TPE. These medications are typically given immediately after, and not prior to, TPE to prevent their removal since TPE can clear protein-bound drugs from the circulation, such as IVIg.
D. TPE is less effective than IVIg and should only be used to treat refractory patients.
Rationale: TPE has been shown to be equally effective as, if not more effective than, IVIg.
E. Use of TPE prior to thymectomy should be avoided due to the removal of coagulation factors.
Rationale: TPE is commonly used prior to thymectomy with improved outcomes in most studies. Most coagulation factor levels are normal within 1 to 2 days after TPE.
Rationale: Prophylaxis for acute cellular rejection after heart transplantation is a category I indication for extracorporeal photopheresis.
B. Transplantation across ABO types is easily managed with TPE and immunosuppression.
Rationale: ABO-incompatible heart transplantation is associated with high rates of hyperacute rejection and early graft failure and is generally avoided in adults.
C. TPE is a first-line therapy for acute cellular rejection.
Rationale: TPE is not used to treat acute cellular rejection.
D. FFP is the replacement fluid of choice during TPE due to the high incidence of coagulopathy.
Rationale: FFP and/or albumin may be used as a replacement fluid.
E. Acute antibody-mediated rejection is most commonly seen in this setting.
Rationale: Acute cellular rejection is more common in this setting.
B. Decreased destruction of HPCs in the peripheral blood.
C. Increased release of HPCs from the bone marrow.
D. Increased maturation of HPCs in the bone marrow.
E. Increased activation of HPCs in the peripheral blood.
Rationale: By enhancing their release from the bone marrow, plerixafor leads to leukocytosis and increased hematopoietic stem cell counts in the peripheral blood.
Rationale: TPE is a second-line treatment or category II indication for desensitization prior to renal transplantation in the setting of a living donor with a positive crossmatch caused by donor-specific HLA antibody(ies).
B. Antibody-mediated rejection of a renal allograft.
Rationale: TPE has been shown to be a first-line therapy, either alone or in conjunction with other immunosuppressive therapy, to treat this type of rejection.
C. Diarrhea-associated hemolytic uremic syndrome.
Rationale: Published evidence suggests that TPE is ineffective in this setting.
D. Immune complex–induced rapidly progressive glomerulonephritis.
Rationale: Published evidence is insufficient to establish a definitive benefit for TPE in this setting.
E. Myeloma kidney.
Rationale: TPE is considered a second-line therapy, either as a standard treatment or in conjunction with other modes of treatment.
Rationale: The use of TPE to decrease RBC alloantibody titers is not recommended in this setting.
B. To decrease donor-specific anti-HLA antibody titers.
Rationale: TPE has been used for decreasing donor-specific HLA antibody titers in solid organ transplants.
C. To decrease donor-specific anti–human platelet antigen (HPA) antibody titers.
Rationale: Although TPE may decrease donor-specific anti-HPA antibody titers, it has not been recommended specifically for this purpose.
D. To decrease complement levels.
Rationale: The use of TPE to decrease complement levels is not recommended in this setting.
E. To decrease donor-specific anti–human neutrophil antigen (HNA) antibody titers.
Rationale: The use of TPE to decrease anti-HNA antibody titers is not recommended in this setting.
Rationale: This patient does not require an RBC transfusion, and platelet transfusions should be avoided in this setting in the absence of life-threatening bleeding.
B. Transfuse 10 U cryoprecipitate.
Rationale: This patient does not need cryoprecipitate in this setting.
C. Transfuse 2 U plasma.
Rationale: If plasmapheresis is unavailable, then transfusion of FFP is the next-best course of action until plasmapheresis can be performed.
D. Initiate a series of plasmapheresis procedures.
Rationale: This is the first-line therapy for patients with TTP and should be initiated as soon as possible.
E. Perform a whole blood exchange.
Rationale: Although this would provide new plasma and would remove autoantibodies, this is not the best course of treatment for this patient.
Rationale: See Major Points of Discussion.
B. Category II indications include diseases and syndromes for which apheresis is the primary treatment in conjunction with another treatment modality.
Rationale: Category II indications includes diseases and syndromes for which apheresis is a second-line therapy.
C. Category III indications include diseases and syndromes for which apheresis is a second-line therapy.
Rationale: Category III indications include diseases and syndromes for which the efficacy of apheresis has not been established.
D. Category IV indications include diseases and syndromes for which apheresis is the last line of treatment.
Rationale: Category IV indications include diseases and syndromes for which apheresis is ineffective or harmful.
E. Category V indications include diseases and syndromes for which apheresis is ineffective or harmful.
Rationale: There is no category V defined in these guidelines.
Rationale: This is the description of a category I indication.
B. Disorder for which apheresis is accepted as second-line therapy, either as a primary stand-alone treatment or in conjunction with other modes of treatment.
Rationale: This is the description of a category II indication. Severe malaria is a category II indication for the use of RBC exchange (i.e., erythrocytapheresis). Antimalarial antimicrobial therapy is the first-line approach for treating malaria.
C. The optimal role of apheresis is not established; decision making should be individualized.
Rationale: This is the description of a category III indication.
D. Disorder in which published evidence demonstrates or suggests that apheresis is ineffective or harmful.
E. Disorder for which approval for a research study is required from an IRB.
Rationale: These are part of the description of a category IV indication.
Rationale: This is the description of a category I indication.
B. Disorder for which apheresis is accepted as second-line therapy, either as a primary stand-alone treatment or in conjunction with other modes of treatment.
Rationale: This is the description of a category II indication.
C. The optimal role of apheresis is not established; decision making should be individualized.
Rationale: This is the description of a category III indication. Thrombotic microangiopathy: hematopoietic stem cell transplant–associated is a category III indication for the use of plasmapheresis. It should be considered as salvage therapy for selected patients with persistent/progressive thrombotic microangiopathy despite resolution of infections and graft-versus-host disease.
D. Disorder in which published evidence demonstrates or suggests that apheresis is ineffective or harmful.
E. Disorder for which approval for a research study is required from an IRB.
Rationale: These are part of the description of category IV indications.
Rationale: An ideal solute resides only in the intravascular space.
B. It is synthesized during the time frame of the plasmapheresis procedure.
Rationale: Ideal solutes are not rapidly synthesized and there is no net synthesis during the time frame of the procedure.
C. It is catabolized during the time frame of the plasmapheresis procedure.
Rationale: Ideal solutes are not rapidly catabolized and there is no net catabolism during the time frame of the procedure.
D. It is completely intravascular.
Rationale: Plasmapheresis directly removes soluble substances only from the intravascular space.
E. It is an IgG antibody.
Rationale: IgG antibodies are predominantly extravascular; thus, these are less efficiently removed by plasmapheresis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree