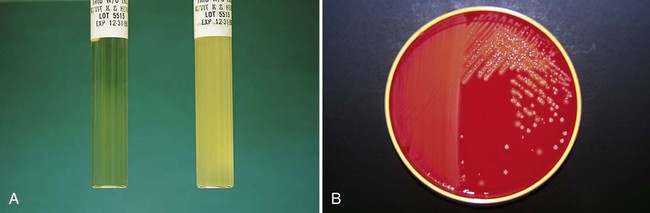
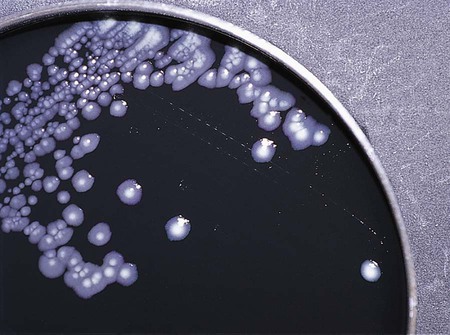
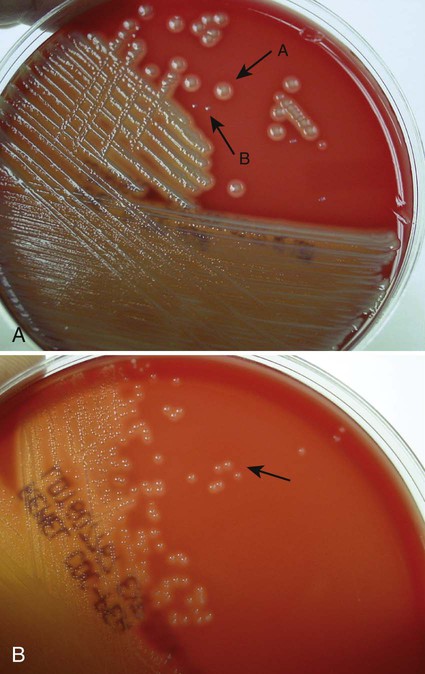
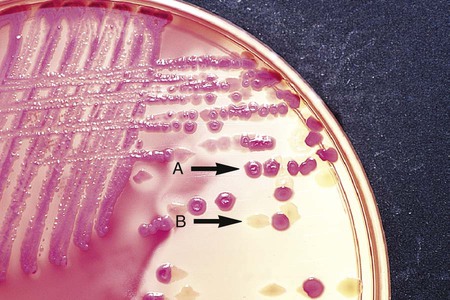
Chapter 7 1. Define bacterial cultivation and list the three most important purposes for bacterial cultivation. 2. Define bacterial media; list the four general types of media and explain the general biochemical principle for each type. 3. List the environmental conditions that are crucial in supporting bacterial in vitro growth and explain how each factor is controlled and monitored. 4. Explain the most common bacterial streaking technique, the principle associated with the technique, and how colonies are enumerated using this technique. 5. Identify the key criteria used in characterizing and reporting bacterial culture growth pertaining to the phenotypic results; differentiate genotypic and phenotypic characteristics. 6. Explain the use and chemical principle of the following enzymatic tests used in preliminary bacterial identification: catalase test, oxidase test, urease test, indole test, PYR test, and hippurate hydrolysis. 7. Define and differentiate bacterial susceptibility and resistance; give an example in how these are used to assist in the identification bacteria. 8. List the three assays used to measure metabolic pathways and provide an example of each. 9. Describe the steps required to develop “rapid” identification schemes and explain how these differ from conventional schemes. 10. List the four basic identification components common to all commercially available multitest systems. • To grow and isolate all bacteria present in a clinical specimen • To determine which of the bacteria that grow are most likely causing infection and which are likely contaminants or colonizers • To obtain sufficient growth of clinically relevant bacteria to allow identification, characterization, and susceptibility testing As discussed in Chapter 2, bacteria have numerous nutritional needs that include different gases, water, various ions, nitrogen, sources for carbon, and energy. The source for carbon and energy is commonly supplied in carbohydrates (e.g., sugars and their derivatives) and proteins. In broth media, nutrients are dissolved in water, and bacterial growth is indicated by a change in the broth’s appearance from clear to turbid (i.e., cloudy). The turbidity, or cloudiness, of the broth is due to light deflected by bacteria present in the culture (Figure 7-1). More growth indicates a higher cell density and greater turbidity. At least 106 bacteria per milliliter of broth are needed for turbidity to be detected with the unaided eye. With appropriate incubation conditions, each bacterial cell inoculated onto the agar medium surface will proliferate to sufficiently large numbers to be observable with the unaided eye (see Figure 7-1). The resulting bacterial population is considered to be derived from a single bacterial cell and is known as a pure colony. In other words, all bacterial cells within a single colony are the same genus and species, having identical genetic and phenotypic characteristics (i.e., are derived from a single clone). Pure cultures are required for subsequent procedures used to identify and characterize bacteria. The ability to select pure (individual) colonies is one of the first and most important steps required for bacterial identification and characterization. Enrichment media contain specific nutrients required for the growth of particular bacterial pathogens that may be present alone or with other bacterial species in a patient specimen. This media type is used to enhance the growth of a particular bacterial pathogen from a mixture of organisms by providing specific nutrients for the organism’s growth. One example of such a medium is buffered charcoal-yeast extract agar, which provides l-cysteine and other nutrients required for the growth of Legionella pneumophila, the causative agent of legionnaires’ disease (Figure 7-2). Nutritive media or supportive media contain nutrients that support growth of most nonfastidious organisms without giving any particular organism a growth advantage. Nutrient media include tryptic soy agar, or nutrient agar plates for bacteria or Sabouraud’s dextrose agar for fungi. Selective media contain one or more agents that are inhibitory to all organisms except those “selected” by the specific growth condition or chemical. In other words, these media select for the growth of certain bacteria to the disadvantage of others. Inhibitory agents used for this purpose include dyes, bile salts, alcohols, acids, and antibiotics. An example of a selective medium is phenylethyl alcohol (PEA) agar, which inhibits the growth of aerobic and facultatively anaerobic gram-negative rods and allows gram-positive cocci to grow (Figure 7-3). Selective and inhibitory chemicals included within nutritive media prevent the overgrowth of normal flora or contaminating organisms that would prevent the identification of pathogenic organisms. However, it is important to note that the use of selective media does not ensure that the inhibited organisms are not present in small quantity and may simply be too small to see. Differential media employ some factor (or factors) that allows colonies of one bacterial species or type to exhibit certain metabolic or culture characteristics that can be used to distinguish it from other bacteria growing on the same agar plate. One commonly used differential medium is MacConkey agar, which differentiates between gram-negative bacteria that can and cannot ferment the sugar lactose (Figure 7-4). Of importance, many media used in diagnostic bacteriology provide more than one function. For example, MacConkey agar is both differential and selective or combination media because it will not allow most gram-positive bacteria to grow. Another example is sheep blood agar. This is the most commonly used nutritive medium for diagnostic bacteriology because it allows many organisms to grow. However, in many ways this agar is also differential because the appearance of colonies produced by certain bacterial species is readily distinguishable, as indicated in Figure 5-2. Figure 7-5 shows differential hemolytic patterns by various organisms. Various broth and agar media that have enrichment, selective, or differential capabilities and are used frequently for routine bacteriology are listed alphabetically in Table 7-1. Anaerobic bacteriology (Section 13), mycobacteriology (Section 14), and mycology (Chapter 60) use similar media strategies; details regarding these media are provided in the appropriate chapters. TABLE 7-1 Plating Media for Routine Bacteriology Brain-heart infusion (BHI) is a nutritionally rich medium used to grow various microorganisms, either as a broth or as an agar, with or without added blood. Key ingredients include infusion from several animal tissue sources, added peptone (protein), phosphate buffer, and a small concentration of dextrose. The carbohydrate provides a readily accessible source of energy for many bacteria. BHI broth is often used as a major component of the media developed for culturing a patient’s blood for bacteria (see Chapter 69), for establishing bacterial identification, and for certain tests to determine bacterial susceptibility to antimicrobial agents (see Chapter 12). Hektoen enteric (HE) agar contains bile salts and dyes (bromthymol blue and acid fuchsin) to selectively slow the growth of most nonpathogenic gram-negative bacilli found in the gastrointestinal tract and allow Salmonella spp. and Shigella spp. to grow. The medium is also differential because many non-enteric pathogens that do grow will appear as orange to salmon-colored colonies. This colony appearance results from the organism’s ability to ferment the lactose in the medium, resulting in the production of acid, which lowers the medium’s pH and causes a change in the pH indicator bromthymol blue. Salmonella spp. and Shigella spp. do not ferment lactose, so no color change occurs and their colonies maintain the original blue-green color of the medium. As an additional differential characteristic, the medium contains ferric ammonium citrate, an indicator for the detection of H2S, so that H2S-producing organisms, such as Salmonella spp., can be visualized as colonies exhibiting a black precipitate (Figure 7-6). MacConkey agar is the most frequently used primary selective and differential agar. This medium contains crystal violet dye to inhibit the growth of gram-positive bacteria and fungi, and it allows many types of gram-negative bacilli to grow. The pH indicator, neutral red, provides this medium with a differential capacity. Bacterial fermentation of lactose results in acid production, which decreases medium pH and causes the neutral red indicator to give bacterial colonies a pink to red color. Non–lactose-fermenters, such as Shigella spp., remain colorless and translucent (see Figure 7-4). Phenylethyl alcohol (PEA) agar is essentially sheep blood agar that is supplemented with phenylethyl alcohol to inhibit the growth of gram-negative bacteria. Five percent sheep blood in PEA provides nutrients for common gram-positive cocci such as enterococci, streptococci, and staphylococci (see Figure 7-3). PEA agar, although it contains sheep blood, should not be used in the interpretation of hemolytic reactions. Gram-negative, facultatively anaerobic bacilli (i.e., those that can grow in the presence or absence of oxygen) generally produce diffuse, even growth throughout the broth, whereas gram-positive cocci demonstrate flocculation or clumps. Strict aerobic bacteria (i.e., require oxygen for growth), such as Pseudomonas spp., tend to grow toward the surface of the broth, whereas strict anaerobic bacteria (i.e., those that cannot grow in the presence of oxygen) grow at the bottom of the broth (Figure 7-7). As with HE agar, xylose-lysine-desoxycholate (XLD) agar is selective and differential for Shigella spp. and Salmonella spp. The salt, sodium desoxycholate, inhibits many gram-negative bacilli that are not enteric pathogens and inhibits gram-positive organisms. A phenol red indicator in the medium detects increased acidity from carbohydrate (i.e., lactose, xylose, and sucrose) fermentation. Enteric pathogens, such as Shigella spp., do not ferment these carbohydrates, so their colonies remain colorless (i.e., the same approximate pink to red color of the un-inoculated medium). Even though they often ferment xylose, colonies of Salmonella spp. are also colorless on XLD, because of the decarboxylation of lysine, which results in a pH increase that causes the pH indicator to turn red. These colonies often exhibit a black center that results from Salmonella spp. producing H2S. Several of the nonpathogenic organisms ferment one or more of the sugars and produce yellow colonies (Figure 7-8). Delicate media components that cannot withstand steam sterilization by autoclaving (e.g., serum, certain carbohydrate solutions, certain antibiotics, and other heat-labile substances) can be sterilized by membrane filtration. Passage of solutions through membrane filters with pores ranging in size from 0.2 to 0.45 µm in diameter will not remove viruses but does effectively remove most bacterial and fungal contaminants. Finally, all media, whether purchased or prepared, must be subjected to stringent quality control before being used in the diagnostic setting (for more information regarding quality control see Chapter 79). The cultures for growth of these bacteria comprise layers of living cells growing on the surface of a solid matrix such as the inside of a glass tube or the bottom of a plastic flask. The presence of bacterial pathogens within the cultured cells is detected by specific changes in the cells’ morphology. Alternatively, specific stains, composed of antibody conjugates, may be used to detect bacterial antigens within the cells. Cell cultures may also detect certain bacterial toxins (e.g., Clostridium difficile cytotoxin). Cell cultures are most commonly used in diagnostic virology. Cell culture maintenance and inoculation is addressed in Chapter 66.
Traditional Cultivation and Identification
Principles of Bacterial Cultivation
Nutritional Requirements
Phases of Growth Media
Media Classifications and Functions
Summary of Artificial Media for Routine Bacteriology
Medium
Components/Comments
Primary Purpose
Bile esculin agar (BEA)
Nutrient agar base with ferric citrate. Hydrolysis of esculin by group D streptococci imparts a brown color to medium; sodium desoxycholate inhibits many bacteria
Differential isolation and presumptive identification of group D streptococci and enterococci
Bile esculin azide agar with vancomycin
Contains azide to inhibit gram-negative bacteria, vancomycin to select for resistant gram-positive bacteria, and bile esculin to differentiate enterococci from other vancomycin-resistant bacteria that may grow
Selective and differential for cultivation of vancomycin-resistant enterococci from clinical and surveillance specimens
Blood agar
Trypticase soy agar, Brucella agar, or beef heart infusion with 5% sheep blood
Cultivation of nonfastidious microorganisms, determination of hemolytic reactions
Bordet-Gengou agar
Potato-glycerol–based medium enriched with 15%-20% defibrinated blood; contaminants inhibited by methicillin (final concentration of 2.5 µm/mL)
Isolation of Bordetella pertussis and Bordetella parapertussis
Brain heart infusion agar or broth
Dextrose, pork brain and heart dehydrated infusions.
Cultivation of fastidious organisms.
Buffered charcoal-yeast extract agar (BCYE)
Yeast extract, agar, charcoal, and salts supplemented with L-cysteine HCl, ferric pyrophosphate, ACES buffer, and α-ketoglutarate
Enrichment for Legionella spp.
Supports the growth of Francisella and Nocardia spp.
Buffered charcoal-yeast extract (BCYE) agar with antibiotics
BCYE supplemented with polymyxin B, vancomycin, and ansamycin, to inhibit gram-negative bacteria, gram-positive bacteria, and yeast, respectively
Enrichment and selection for Legionella spp.
Burkholderia cepacia selective agar
Bile salts, gentamycin, ticarcillin, polymixin B, Peptone, yeast extract
For recovery of B. Cepacia from cystic fibrosis patients
Campy-blood agar
Contains vancomycin (10 mg/L), trimethoprim (5 mg/L), polymyxin B (2500 U/L), amphotericin B (2 mg/L), and cephalothin (15 mg/L) in a Brucella agar base with sheep blood
Selective for Campylobacter spp.
Campylobacter thioglycollate broth
Thioglycollate broth supplemented with increased agar concentration and antibiotics
Selective holding medium for recovery of Campylobacter spp.
Incubated at 4° C for cold-enrichment.
CDC anaerobe 5% sheep blood agar
Tryptic soy broth, 5% sheep blood and added nutrients
Improved growth of obligate, slow-growing anaerobes
Cefoperazone, vancomycin, amphotericin (CVA) medium
Blood-supplemented enrichment medium containing cefoperazone, vancomycin, and amphotericin to inhibit growth of most gram-negative bacteria, gram-positive bacteria, and yeast, respectively
Selective medium for isolation of Campylobacter spp.
Cefsulodin-irgasan-novobiocin (CIN) agar
Peptone base with yeast extract, mannitol, and bile salts; supplemented with cefsulodin, irgasan, and novobiocin; neutral red and crystal violet indicators
Selective for Yersinia spp.; may be useful for isolation of Aeromonas spp.
Chocolate agar
Peptone base, enriched with solution of 2% hemoglobin or IsoVitaleX (BBL)
Cultivation of fastidious microorganisms such as Haemophilus spp., Brucella spp. and pathogenic Neisseria spp.
Chromogenic media
Organism-specific nutrient base, selective supplements and chromogenic substrate
Chromogenic media are designed to optimize growth and differentiate a specific type of organism. Chromagars are routinely used in the identification of yeasts, methicillin-resistant Stapylococcus aureus (MRSA), and a variety of other organisms.
Columbia colistin-nalidixic acid (CNA) agar
Columbia agar base with 10 mg colistin per liter, 15 mg nalidixic acid per liter, and 5% sheep blood
Selective isolation of gram-positive cocci
Cystine-tellurite blood agar
Infusion agar base with 5% sheep blood; reduction of potassium tellurite by Corynebacterium diphtheriae produces black colonies
Isolation of C. diphtheriae
Eosin methylene blue (EMB) agar (Levine)
Peptone base containing lactose; eosin Y and methylene blue as indicators
Isolation and differentiation of lactose-fermenting and non–lactose-fermenting enteric bacilli
Gram-negative broth (GN)
Peptone base broth with glucose and mannitol; sodium citrate and sodium desoxycholate act as inhibitory agents
Selective (enrichment) liquid medium for enteric pathogens
Hektoen enteric (HE) agar
Peptone base agar with bile salts, lactose, sucrose, salicin, and ferric ammonium citrate; indicators include bromthymol blue and acid fuchsin
Differential, selective medium for the isolation and differentiation of Salmonella and Shigella spp. from other gram-negative enteric bacilli
Loeffler’s medium
Animal tissue (heart muscle), dextrose, eggs and beef serum, and sodium chloride
Isolation and growth of Corynebacterium
MacConkey agar
Peptone base with lactose; gram-positive organisms inhibited by crystal violet and bile salts; neutral red as indicator
Isolation and differentiation of lactose fermenting and non–lactose-fermenting enteric bacilli
MacConkey sorbitol agar
A modification of MacConkey agar in which lactose has been replaced with d-sorbitol as the primary carbohydrate
For the selection and differentiation of E. coli O157:H7 in stool specimens
Mannitol salt agar
Peptone base, mannitol, and phenol red indicator; salt concentration of 7.5% inhibits most bacteria
Selective differentiation of staphylococci
New York City (NYC) agar
Peptone agar base with cornstarch, supplemented with yeast dialysate, 3% hemoglobin, and horse plasma; antibiotic supplement includes vancomycin (2 µg/mL), colistin (5.5 µg/mL), amphotericin B (1.2 µg/mL), and trimethoprim (3 µg/mL)
Selective for Neisseria gonorrhoeae;
also supports the growth of Ureaplasma urealyticum and some Mycoplasma spp.
Phenylethyl alcohol (PEA) agar
Nutrient agar base. Phenylmethanol inhibits growth of gram-negative organisms
Selective isolation of aerobic gram-positive cocci and bacilli and anaerobic gram-positive cocci and negative bacilli
Regan Lowe
Charcoal agar supplemented with horse blood, cephalexin, and amphotericin B
Enrichment and selective medium for isolation of Bordetella pertussis
Salmonella-Shigella (SS) agar
Peptone base with lactose, ferric citrate, and sodium citrate; neutral red as indicator; inhibition of coliforms by brilliant green and bile salts
Selective for Salmonella and some Shigella spp.
Schaedler agar
Peptone and soy protein base agar with yeast extract, dextrose, and buffers; addition of hemin, l-cystine, and 5% blood enriches for anaerobes
Nonselective medium for the recovery of anaerobes and aerobes
Selective for Campylobacter and Helicobacter spp.
Selenite broth
Peptone base broth; sodium selenite toxic for most Enterobacteriaceae
Enrichment of isolation of Salmonella spp.
Skirrow agar
Peptone and soy protein base agar with lysed horse blood; vancomycin inhibits gram-positive organisms; polymyxin B and trimethoprim inhibit most gram-negative organisms
Selective for Campylobacter spp.
Streptococcal selective agar (SSA)
Contains crystal violet, colistin, and trimethoprim- sulfamethoxazole in 5% sheep blood agar base
Selective for Streptococcus pyogenes and Streptococcus agalactiae
Tetrathionate broth
Peptone base broth; iodine and potassium iodide, bile salts, and sodium thiosulfate inhibit gram-positive organisms and Enterobacteriaceae
Selective for Salmonella and Shigella spp. except S. typhi.
Thayer-Martin agar
(modified Thayer Martin)
Blood agar base enriched with hemoglobin and supplement B; contaminating organisms inhibited by colistin, nystatin, vancomycin, and trimethoprim
Selective for N. gonorrhoeae and N. meningitidis.
Supports the growth of Francisella and Brucella spp.
Thioglycollate broth
Pancreatic digest of casein, soy broth, and glucose enrich growth of most microorganisms; includes reducing agents thioglycolate, cystine, and sodium sulfite; semisolid medium with a low concentration of agar reducing oxygen diffusion in the medium
Supports growth of anaerobes, aerobes, microaerophilic, and fastidious microorganisms
Thiosulfate citrate-bile salts (TCBS) agar
Peptone base agar with yeast extract, bile salts, citrate, sucrose, ferric citrate, and sodium thiosulfate; bromthymol blue acts as indicator
Selective and differential for Vibrio spp.
Todd-Hewitt broth supplemented with antibiotics (LIM)
Todd-Hewitt, an enrichment broth for streptococci, is supplemented with nalidixic acid and gentamicin or colistin for greater selectivity; thioglycollate and agar reduce redox potential
Selection and enrichment for Streptococcus agalactiae in female genital specimens
Trypticase soy broth (TSB)
All-purpose enrichment broth that can support the growth of many fastidious and nonfastidious bacteria
Enrichment broth used for subculturing various bacteria from primary agar plates
Xylose lysine desoxycholate (XLD) agar
Yeast extract agar with lysine, xylose, lactose, sucrose, and ferric ammonium citrate; sodium desoxycholate inhibits gram-positive organisms; phenol red as indicator
Isolation and differentiation of Salmonella and Shigella spp. from other gram-negative enteric bacilli
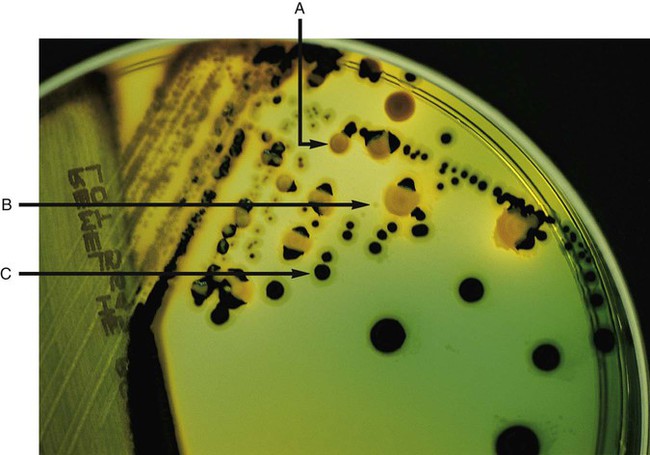
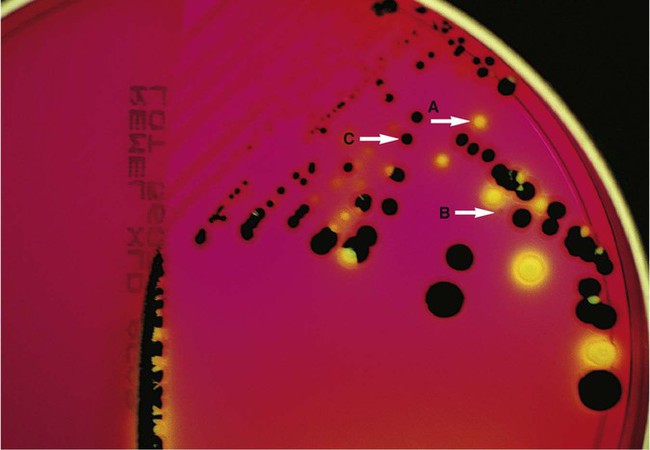
Brain-Heart Infusion.
Hektoen Enteric (HE) Agar.
MacConkey Agar.
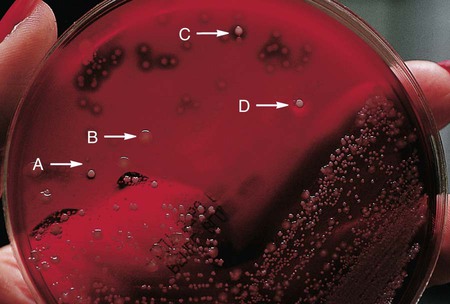
Phenylethyl Alcohol (PEA) Agar.
Thioglycollate Broth.
Xylose-Lysine-Desoxycholate (XLD) Agar.
Preparation of Artificial Media
Media Sterilization.
Cell Cultures.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Traditional Cultivation and Identification