FIGURE 22-1 When intravenous theophylline or aminophylline is administered to a patient as a continuous infusion, it will take 3-5 half-lives for serum theophylline concentrations to reach steady-state levels. Because of this, maximal drug response will take time to achieve. To hasten onset of drug action, loading doses are given to attain effective theophylline concentrations immediately.
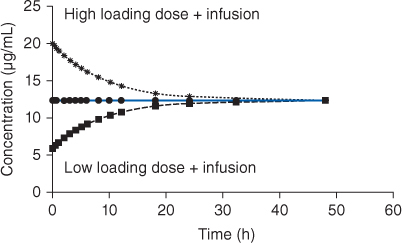
FIGURE 22-2 If the patient’s own, unique theophylline volume of distribution (V) is known, the exact loading dose (LD) of intravenous theophylline or aminophylline to immediately achieve steady-state theophylline concentrations (Css) can be calculated (LD = Css • V). However, the volume of distribution for the patient is rarely known when loading doses need to be administered, and, for practical purposes, an average population volume of distribution for theophylline is used to estimate the parameter for the patient (V = 0.5 L/kg, use ideal body weight if >30% overweight). Because of this, the computed loading dose will almost always be too large or too small to reach the desired steady-state theophylline concentration, and it will still take 3-5 half-lives to attain steady-state conditions.
After an efficacious theophylline dosage regimen has been established for a patient, theophylline serum concentrations remain fairly stable in patients receiving long-term therapy. In these cases, theophylline dosage requirements and steady-state serum concentrations should be reassessed on a yearly basis. In patients with congestive heart failure or liver cirrhosis, theophylline dosage requirements can vary greatly according to the status of the patient. For example, if a patient with compensated heart failure is receiving a stable dose of theophylline, but experiences an exacerbation of their heart disease, it is very likely that they will need to have their theophylline dosage requirements reassessed to avoid theophylline toxicity. Also, acute viral diseases, especially in children, have been associated with theophylline adverse effects in patients previously stabilized on effective, nontoxic theophylline dosage regimens.10,11 Methods to adjust theophylline doses using serum concentrations are discussed later in this chapter.
BASIC CLINICAL PHARMACOKINETIC PARAMETERS
Theophylline is primarily eliminated by hepatic metabolism (>90%). Hepatic metabolism is mainly via the CYP1A2 enzyme system with a smaller amount metabolized by CYP3A and CYP2E1. About 10% of a theophylline dose is recovered in the urine as unchanged drug.12,13 Strictly speaking, theophylline follows non-linear pharmacokinetics.14–16 However, for the purposes of clinical drug dosing in patients, linear pharmacokinetic concepts and equations can be effectively used to compute doses and estimate serum concentrations. Occasionally, theophylline serum concentrations increase in a patient more than expected after a dosage increase for an unidentifiable reason, and nonlinear pharmacokinetics may explain the observation.14–16
Three different forms of theophylline are available. Aminophylline is the ethylenediamine salt of theophylline, and anhydrous aminophylline contains about 85% theophylline while aminophylline dihydrate contains about 80% theophylline. Oxtriphylline is the choline salt of theophylline and contains about 65% theophylline. Theophylline and aminophylline are available for intravenous injection and oral use. Oxtriphylline is available only for oral use. The oral bioavailability of all three theophylline-based drugs is very good and generally equals 100%. However, some older sustained-release oral dosage forms have been reported to exhibit incomplete bioavailability and loss of slow release characteristics under certain circumstances due to their tablet or capsule design. Theophylline plasma protein binding is only 40%.17,18
The recommended dose of theophylline or one of its salt forms is based on the concurrent disease states and conditions present in the patient that can influence theophylline pharmacokinetics. Theophylline pharmacokinetic parameters used to compute doses are given in the following section for specific patient profiles.
EFFECTS OF DISEASE STATES AND CONDITIONS ON THEOPHYLLINE PHARMACOKINETICS AND DOSING
Normal adults without the disease states and conditions given later in this section with normal liver function have an average theophylline half-life of 8 hours (range: 6-12 hours) and volume of distribution of 0.5 L/kg (range: 0.4-0.6 L/kg; Table 22-1).19–21 Most disease states and conditions that change theophylline pharmacokinetics and dosage requirements alter clearance, but volume of distribution remains stable at ~0.5 L/kg in these situations. Tobacco and marijuana smoke causes induction of hepatic CYP1A2 which accelerates the clearance of theophylline.19–24 In patients who smoke these substances, the average theophylline half-life is 5 hours. When patients stop smoking these compounds, theophylline clearance slowly approaches its baseline level for the patient over a 6- to 12-month period if the patient does not encounter “second-hand” smoke produced by other users.25 If the patient inhales a sufficient amount of second-hand smoke, theophylline clearance for the exsmoker may remain in the fully induced state or at some intermediate induced state.26
TABLE 22-1 Disease States and Conditions That Alter Theophylline Pharmacokinetics
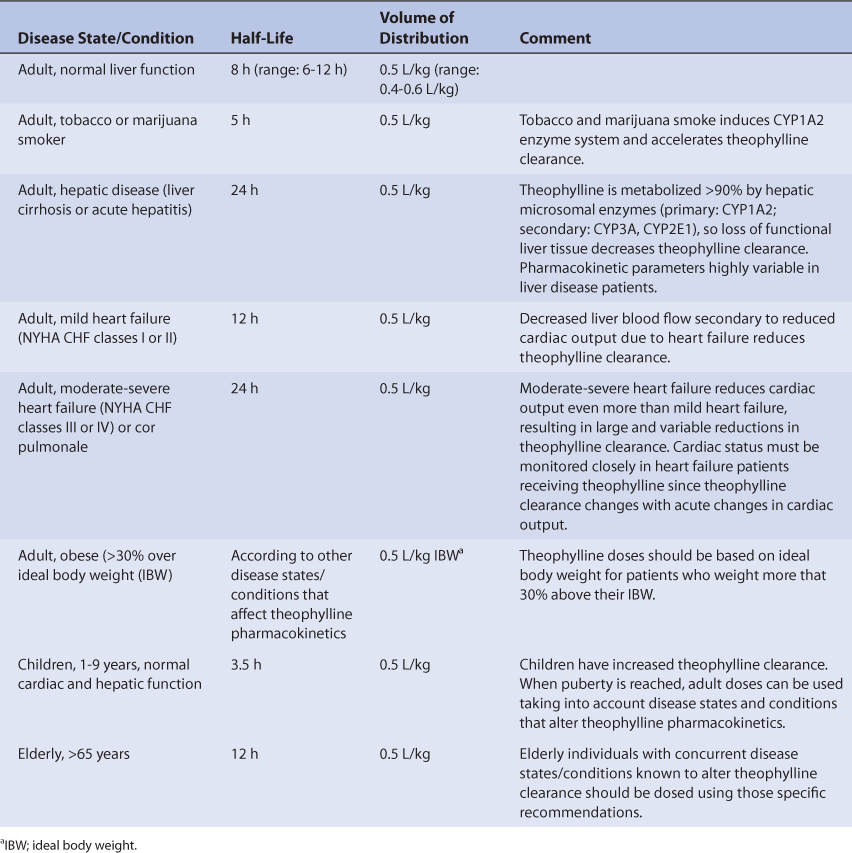
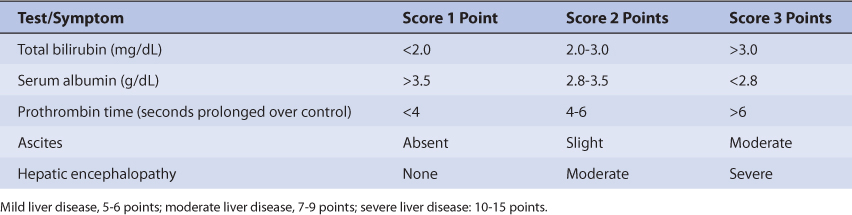
Patients with liver cirrhosis or acute hepatitis have reduced theophylline clearance which results in a prolonged average theophylline half-life of 24 hours.20,27–29 However, the effect that liver disease has on theophylline pharmacokinetics is highly variable and difficult to accurately predict. It is possible for a patient with liver disease to have relatively normal or grossly abnormal theophylline clearance and half-life. For example, a liver disease patient who also smokes cigarettes could have a theophylline half-life equal to 5 hours if some liver parenchyma is present and tobacco-induced enzyme induction occurred, or 50 hours if little or no liver tissue remains. An index of liver dysfunction can be gained by applying the Child-Pugh clinical classification system to the patient (Table 22-2). Child-Pugh scores are completely discussed in Chapter 3 (Drug Dosing in Special Populations: Renal and Hepatic Disease, Dialysis, Heart Failure, Obesity, and Drug Interactions), but will be briefly discussed here. The Child-Pugh score consists of five laboratory tests or clinical symptoms: serum albumin, total bilirubin, prothrombin time, ascites, and hepatic encephalopathy. Each of these areas is given a score of 1 (normal) to 3 (severely abnormal; see Table 22-2), and the scores for the five areas are summed. The Child-Pugh score for a patient with normal liver function is 5 while the score for a patient with grossly abnormal serum albumin, total bilirubin, and prothrombin time values in addition to severe ascites and hepatic encephalopathy is 15. A Child-Pugh score greater than 8 is grounds for a decrease in the initial daily drug dose for theophylline (t1/2 = 24 hours). As in any patient with or without liver dysfunction, initial doses are meant as starting points for dosage titration based on patient response and avoidance of adverse effects. Theophylline serum concentrations and the presence of adverse drug effects should be monitored frequently in patients with liver cirrhosis.
TABLE 22-2 Child-Pugh Scores for Patients With Liver Disease77
Heart failure causes reduced theophylline clearance because of decreased hepatic blood flow secondary to compromised cardiac output.20,30–33 Venous stasis of blood within the liver may also contribute to the decrease in theophylline clearance found in heart failure patients. Patients with mild heart failure (New York Heart Association or NYHA Class I or II, Table 22-3) have an average theophylline half-life equal to 12 hours (range: 5-24 hours) while those with moderate to severe heart failure (NYHA Class III or IV) or cor pulmonale have an average theophylline half-life of 24 hours (5-50 hours). Obviously, the effect that heart failure has on theophylline pharmacokinetics is highly variable and difficult to accurately predict. It is possible for a patient with heart failure to have relatively normal or grossly abnormal theophylline clearance and half-life. For heart failure patients, initial doses are meant as starting points for dosage titration based on patient response and avoidance of adverse effects. Theophylline serum concentrations and the presence of adverse drug effects should be monitored frequently in patients with heart failure.
TABLE 22-3 New York Heart Association (NYHA) Functional Classification for Heart Failure78
Obese patients (>30% above ideal body weight or IBW) should have volume of distribution estimates based on ideal body weight.34–37 Theophylline half-life should be based on the concurrent disease states and conditions present in the patient. If weight-based dosage recommendations (mg/kg/d or mg/kg/h) are to be used, ideal body weight should be used to compute doses for obese individuals.
Patient age has an effect on theophylline clearance and half-life. Newborns have decreased theophylline clearance because hepatic drug–metabolizing enzymes are not yet fully developed at birth. Premature neonates have average theophylline half-lives equal to 30 hours 3-15 days after birth and 20 hours 25-57 days after birth.38–40 Full-term infants have average theophylline half-lives of 25 hours 1-2 days after birth, and 11 hours 3-30 weeks after birth.41–43 Children between the ages of 1 and 9 years have accelerated theophylline clearance rates resulting in an average half-life of 3.5 hours (range: 1.5-5 hours).44–46 As children achieve puberty, their theophylline clearance and half-life approach the values of an adult. For elderly patients over the age of 65, some studies indicate that theophylline clearance and half-life are the same as in younger adults while other investigations have found that theophylline clearance is slower and half-life is longer (average half-life = 12 hours, range: 8-16 hours).47–51 A confounding factor found in theophylline pharmacokinetic studies conducted in older adults is the possible accidental inclusion of subjects that have subclinical or mild cases of the disease states associated with reduced theophylline clearance (heart failure, liver disease, etc). Thus, the pharmacokinetics of theophylline in elderly individuals is somewhat controversial.
Febrile illnesses can temporarily decrease the clearance of theophylline and require an immediate dosage decrease to avoid toxicity.10,11 The mechanism of this acute change in theophylline disposition is unclear, but probably involves decreased clearance due to the production of interleukins. Children seem to be at an especially high risk of theophylline adverse reactions since febrile illnesses are prevalent in this population and high theophylline doses (on a mg/kg/d basis) are prescribed.
Because only a small amount of theophylline is eliminated unchanged in the urine (<10% of a dose), dosage adjustments are not necessary in patients with renal impairment.12,13 Theophylline is removed by hemodialysis, and, if possible, doses should be held until after the dialysis procedure is complete.52–57 If a pulmonary exacerbation occurs due to decreased theophylline concentrations, individualized supplemental doses of theophylline may need to be given during or after the procedure is complete. The hemoperfusion sieving coefficient for theophylline is 0.80, which indicates significant removal by these techniques.58,59 Theophylline is not appreciably removed by peritoneal dialysis.55
Hypothyroid patients have decreased basal metabolic rates, and require smaller theophylline doses until a euthyroid condition is established.60 The breast milk to serum ratio for theophylline is 0.7.61
DRUG INTERACTIONS
Drug interactions with theophylline are common and occur with a variety of medications.62 Serious inhibition drug interactions are those that decrease theophylline clearance more than 30%. Clinicians should consider an arbitrary decrease in theophylline dose of 30%-50% for patients receiving these agents until the actual degree of hepatic enzyme inhibition can be assessed using theophylline serum concentration monitoring. Patients should also be actively monitored for the signs and symptoms of theophylline toxicity. It should be emphasized that the magnitude of hepatic enzyme inhibition drug interactions is highly variable so some patients may require even larger theophylline dosage decreases while others will exhibit no drug interaction at all. Cimetidine given at higher doses (≥1000 mg/d) on a multiple daily dosage schedule decreases theophylline clearance by 30%-50%. Other cimetidine doses (≤800 mg/d) given once or twice daily decrease theophylline clearance by 20% or less.63,64 Ciprofloxacin and enoxacin, both quinolone antibiotics, and troleandomycin, a macrolide antibiotic, also decrease theophylline clearance by 30%-50%. Estrogen and estrogen-containing oral contraceptives, propranolol, metoprolol, mexiletine, propafenone, pentoxifylline, ticlopidine, tacrine, thiabendazole, disulfiram, nefazodone, interferon, zileuton, and fluvoxamine can also decrease theophylline clearance by this extent.
Moderate-sized inhibition drug interactions are those that decrease theophylline clearance by 10%-30%. For this magnitude of drug interaction, many clinicians believe that a routine decrease in theophylline dose is unnecessary for patients with steady-state theophylline concentrations less than 15 μg/mL, but should be considered on a case-by-case basis for those with concentrations above this level. Should a decrease be warranted in a patient, theophylline doses can be cut by 20% to avoid adverse effects. Again, patients should be actively monitored for the signs and symptoms of theophylline toxicity. The calcium channel blockers, verapamil and diltiazem, have been reported to cause decreases in theophylline clearance of 15%-25%. Clarithromycin and erythromycin, both macrolide antibiotics, and norfloxacin, a quinolone antibiotic, can also decrease theophylline clearance by this magnitude. At doses of 600 mg/d or above, allopurinol has been reported to decrease theophylline clearance by 25%.
Theophylline elimination is also subject to induction of hepatic microsomal enzymes which increases theophylline clearance. Because hepatic microsomal enzyme induction is quite variable in patients, some individuals may require theophylline dosage increases while others will require no alteration in dosage requirements. Also, hepatic microsomal enzyme induction takes time to occur, and maximal effects may not be seen for 2-4 weeks of treatment with enzyme inducers. Patients treated with a drug that increases theophylline clearance need to be carefully monitored for the signs and symptoms of their respective disease state, and steady-state theophylline concentrations should be measured. Disease exacerbations may be due to decreased theophylline concentrations, and a dosage increase may be warranted in some patients. Phenytoin, carbamazepine, phenobarbital, rifampin, and moricizine all increase theophylline clearance.
INITIAL DOSAGE DETERMINATION METHODS
Several methods to initiate theophylline therapy are available. The Pharmacokinetic Dosing method is the most flexible of the techniques. It allows individualized target serum concentrations to be chosen for a patient, and each pharmacokinetic parameter can be customized to reflect specific disease states and conditions present in the patient. Literature-based recommended dosing is a very commonly used method to prescribe initial doses of theophylline. Doses are based on those that commonly produce steady-state concentrations in the lower end of the therapeutic range, although there is a wide variation in the actual concentrations for a specific patient.
Pharmacokinetic Dosing Method
The goal of initial dosing of theophylline is to compute the best dose possible for the patient given their set of disease states and conditions that influence theophylline pharmacokinetics and the pulmonary disorder being treated. In order to do this, pharmacokinetic parameters for the patient will be estimated using average parameters measured in other patients with similar disease state and condition profiles.
Half-Life and Elimination Rate Constant Estimate
Theophylline is predominately metabolized by liver. Unfortunately, there is no good way to estimate the elimination characteristics of liver-metabolized drugs using an endogenous marker of liver function in the same manner that serum creatinine and estimated creatinine clearance are used to estimate the elimination of agents that are renally eliminated. Because of this, a patient is categorized according to the disease states and conditions that are known to change theophylline half-life, and the half-life previously measured in these studies is used as an estimate of the current patient’s half-life. For example, for a patient with COPD who currently smokes tobacco-containing cigarettes, theophylline half-life would be assumed to equal 5 hours. Alternatively, for a patient with moderate heart failure (NYHA CHF class III), theophylline half-life would be assumed to equal 24 hours, while a patient with severe liver disease (Child-Pugh score = 12) would be assigned an estimated half-life of 24 hours. To produce the most conservative theophylline doses in patients with multiple concurrent disease states or conditions that affect theophylline pharmacokinetics, the disease state or condition with the longest half-life should be used to compute doses. This approach will avoid accidental overdosage as much as currently possible. For instance, for a patient with asthma who currently smokes tobacco-containing cigarettes and has severe liver disease, an estimated theophylline half-life of 24 hours would be used to compute initial dosage requirements. Once the correct half-life is identified for the patient, it can be converted into the theophylline elimination rate constant (k) using the following equation: k = 0.693/t1/2.
Volume of Distribution Estimate
Theophylline volume of distribution is relatively stable in patients regardless of the disease states and conditions that are present. Volume of distribution is assumed to equal 0.5 L/kg for nonobese patients. For obese patients (>30% above ideal body weight), ideal body weight is used to compute theophylline volume of distribution. Thus, for an 80-kg patient, the estimated theophylline volume of distribution would be 40 L: V = 0.5 L/kg • 80 kg = 40 L. For a 150-kg obese patient with an ideal body weight of 60 kg, the estimated theophylline volume of distribution is 30 L: V = 0.5 L/kg • 60 kg = 30 L.
Selection of Appropriate Pharmacokinetic Model and Equations
When given by continuous intravenous infusion or orally, theophylline follows a one-compartment pharmacokinetic model (Figures 22-1, 22-3, 22-4). When oral therapy is required, most clinicians utilize a sustained-release dosage form that has good bioavailability (F = 1), supplies a continuous release of theophylline into the gastrointestinal tract, and provides a smooth theophylline serum concentration-time curve that emulates an intravenous infusion after once or twice daily dosing. Because of this, a very simple pharmacokinetic equation that computes the average theophylline steady-state serum concentration (Css in μg/mL = mg/L) is widely used and allows maintenance dosage calculation: Css = [F • S (D/τ)]/Cl or D = (Css • Cl • τ)/(F • S), where F is the bioavailability fraction for the oral dosage form (F = 1 for most oral theophylline sustained-release products); S is the fraction of the theophylline salt form that is active theophylline (S = 1 for theophylline, S = 0.85 for anhydrous aminophylline, S = 0.80 for aminophylline dihydrate, S = 0.65 for oxtriphylline); D is the dose of theophylline salt in mg; and τ is the dosage interval in hours. Cl is theophylline clearance in L/h and is computed using estimates of theophylline elimination rate constant (k) and volume of distribution: Cl = kV. For example, for a patient with an estimated elimination rate constant equal to 0.139 h–1 and an estimated volume of distribution equal to 35 L, the estimated clearance would equal 4.87 L/h: Cl = 0.139h–1 • 35 L = 4.87 L/h.
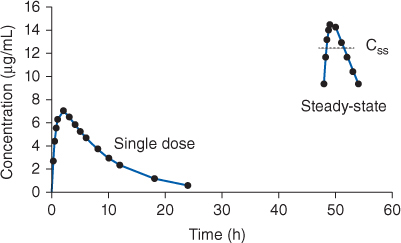
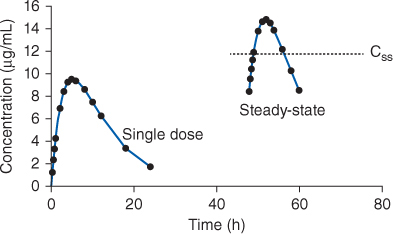
FIGURE 22-3 Serum concentration-time profile for rapid-release theophylline or aminophylline oral dosage forms after a single dose and at steady-state (given every 6 hours). The curves shown would be typical for an adult cigarette smoker receiving theophylline 300 mg. The steady-state serum concentration (Css) expected from an equivalent theophylline or aminophylline continuous infusion is shown by the dotted line in the steady-state concentrations.
FIGURE 22-4 Serum concentration-time profile for sustained-release theophylline or aminophylline oral dosage forms after a single dose and at steady-state (given every 12 hours). The curves shown would be typical for an adult cigarette smoker receiving theophylline 600 mg. The steady-state serum concentration (Css) expected from an equivalent theophylline or aminophylline continuous infusion is shown by the dotted line in the steady-state concentrations.
When intravenous therapy is required, a similar pharmacokinetic equation that computes the theophylline steady-state serum concentration (Css in μg/mL = mg/L) is widely used and allows dosage calculation for a continuous infusion: Css = [S • k0]/Cl or k0 = (Css • Cl)/S, where S is the fraction of the theophylline salt form that is active theophylline (S = 1 for theophylline, S = 0.85 for anhydrous aminophylline, S = 0.80 for aminophylline dihydrate) and k0 is the dose of theophylline salt in mg. Cl is theophylline clearance in L/h and is computed using estimates of theophylline elimination rate constant (k) and volume of distribution: Cl = kV.
The equation used to calculate an intravenous loading dose (LD in mg) is based on a simple, one-compartment model: LD = (Css • V)/S, where Css is the desired theophylline steady-state concentration in μg/mL which is equivalent to mg/L, V is the theophylline volume of distribution, and S is the fraction of the theophylline salt form that is active theophylline (S = 1 for theophylline, S = 0.85 for anhydrous aminophylline, S = 0.80 for aminophylline dihydrate). Intravenous theophylline loading doses should be infusions over at least 20-30 minutes.
Steady-State Concentration Selection
The generally accepted therapeutic ranges for theophylline are 10-20 μg/mL for the treatment of asthma or chronic obstructive pulmonary disease, or 6-13 μg/mL for the treatment of premature apnea. Recent guidelines suggest that for initial treatment of pulmonary disease, clinical response to theophylline concentrations between 5 and 15 μg/mL should be assessed before higher concentrations are used.65 Many patients requiring chronic theophylline therapy will derive sufficient bronchodilatory response with a low likelihood of adverse effects at concentrations of 8-12 μg/mL. However, theophylline therapy much be individualized for each patient in order to achieve optimal responses and minimal side effects.
To illustrate the differences and similarities between oral and intravenous theophylline dosage regimen design, the same cases will be used to compute intravenous theophylline loading doses and continuous infusions.
Literature-Based Recommended Dosing
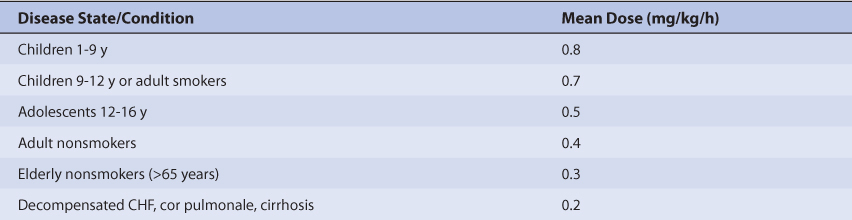
Because of the large amount of variability in theophylline pharmacokinetics, even when concurrent disease states and conditions are identified, many clinicians believe that the use of standard theophylline doses for various situations is warrented.20,66,67 The original computation of these doses was based on the pharmacokinetic dosing method described in the previous section, and subsequently modified based on clinical experience. In general, the expected theophylline steady-state serum concentration used to compute these doses was 10 μg/mL. Suggested theophylline maintenance doses stratified by disease states and conditions known to alter theophylline pharmacokinetics are given in Table 22-4.67 For obese individuals (>30% over ideal body weight), ideal body weight should be used to compute doses.34–37 Because the doses are given in terms of theophylline, doses for other theophylline salt forms need to be adjusted accordingly (S = 1 for theophylline, S = 0.85 for anhydrous aminophylline, S = 0.8 for aminophylline dihydrate, S = 0.65 for oxtriphylline). If theophylline is to be given orally, the dose given in Table 22-4 (in mg/kg/h) must be multiplied by the appropriate dosage interval for the dosage form being used: D = (theophylline dose • Wt • τ)/S, where Wt is patient weight, τ is the dosage interval, and S is the appropriate salt form correction factor for aminophylline or oxtriphylline. If theophylline is to be given as a continuous intravenous infusion the following equation is used to compute the infusion rate: k0 = (theophylline dose • Wt)/S, where Wt is patient weight and S is the appropriate salt form correction factor for aminophylline. When more than one disease state or condition is present in a patient, choosing the lowest dose suggested by Table 22-4 will result in the safest, most conservative dosage recommendation. If an intravenous loading dose is necessary, theophylline 5 mg/kg or aminophylline 6 mg/kg is used; ideal body weight is used to compute loading doses for obese patients (>30% over ideal body weight).
TABLE 22-4 Theophylline Dosage Rates for Patients With Various Disease States and Conditions67
To illustrate the similarities and differences between this method of dosage calculation and the pharmacokinetic dosing method, the same examples used in the previous section will be used.
To illustrate the differences and similarities between oral and intravenous theophylline dosage regimen design, the same cases will be used to compute intravenous theophylline loading doses and continuous infusions.
USE OF THEOPHYLLINE SERUM CONCENTRATIONS TO ALTER DOSES
Because of the large amount of pharmacokinetic variability among patients, it is likely that doses computed using patient population characteristics will not always produce theophylline serum concentrations that are expected or desirable. Because of pharmacokinetic variability, the narrow therapeutic index of theophylline, and the severity of theophylline adverse side effects, measurement of theophylline serum concentrations is mandatory for patients to ensure that therapeutic, nontoxic levels are present. In addition to theophylline serum concentrations, important patient parameters (pulmonary function tests, clinical signs and symptoms of the pulmonary disease state, potential theophylline side effects, etc) should be followed to confirm that the patient is responding to treatment and not developing adverse drug reactions.
When theophylline serum concentrations are measured in patients and a dosage change is necessary, clinicians should seek to use the simplest, most straightforward method available to determine a dose that will provide safe and effective treatment. In most cases, a simple dosage ratio can be used to change theophylline doses assuming the drug follows linear pharmacokinetics. Although it has been clearly demonstrated in research studies that theophylline follows nonlinear pharmacokinetics,14–16 in the clinical setting most patients’ steady-state serum concentrations change in proportion to theophylline dose below and within the therapeutic range, and assuming linear pharmacokinetics is adequate for dosage adjustments in most patients.
Sometimes, it is useful to compute theophylline pharmacokinetic constants for a patient and base dosage adjustments on these. In this case, it may be possible to calculate and use pharmacokinetic parameters to alter the theophylline dose. In some situations, it may be necessary to compute theophylline clearance for the patient during a continuous infusion before steady-state conditions occur using the Chiou method and utilize this pharmacokinetic parameter to calculate the best drug dose.
Finally, computerized methods that incorporate expected population pharmacokinetic characteristics (Bayesian pharmacokinetic computer programs) can be used in difficult cases where serum concentrations are obtained at suboptimal times or the patient was not at steady-state when serum concentrations were measured. An additional benefit of this method is that a complete pharmacokinetic workup (determination of clearance, volume of distribution, and half-life) can be done with one or more measured concentrations that do not have to be at steady-state.
Linear Pharmacokinetics Method
Because theophylline follows linear, dose-proportional pharmacokinetics in most patients with concentrations within and below the therapeutic range, steady-state serum concentrations change in proportion to dose according to the following equation: Dnew/Css,new = Dold/Css,old or Dnew = (Css,new/Css,old)Dold, where D is the dose, Css is the steady-state concentration, old indicates the dose that produced the steady-state concentration that the patient is currently receiving, and new denotes the dose necessary to produce the desired steady-state concentration. The advantages of this method are that it is quick and simple. The disadvantages are steady-state concentrations are required, and the assumption of linear pharmacokinetics may not be valid in all patients. When steady-state serum concentrations increase more than expected after a dosage increase or decrease less than expected after a dosage decrease, nonlinear theophylline pharmacokinetics is a possible explanation for the observation. Because of this, suggested dosage increases greater than 75% using this method should be scrutinized by the prescribing clinician, and the risk versus benefit for the patient assessed before initiating large dosage increases (>75% over current dose).
To illustrate the differences and similarities between oral and intravenous theophylline dosage regimen design, the same cases will be used to compute altered intravenous theophylline continuous infusions using steady-state serum concentrations.
Pharmacokinetic Parameter Method
The Pharmacokinetic Parameter method of adjusting drug doses was among the first techniques available to change doses using serum concentrations. It allows the computation of an individual’s own, unique pharmacokinetic constants and uses those to calculate a dose that achieves desired theophylline concentrations. The Pharmacokinetic Parameter method requires that steady-state has been achieved and uses only a steady-state theophylline concentration (Css). During a continuous intravenous infusion, the following equation is used to compute theophylline clearance (Cl): Cl = [S • k0]/Css, where S is the fraction of the theophylline salt form that is active theophylline (S = 1 for theophylline, S = 0.85 for anhydrous aminophylline, S = 0.80 for aminophylline dihydrate) and k0 is the dose of theophylline salt in mg/h. If the patient is receiving oral theophylline therapy, theophylline clearance (Cl) can be calculated using the following formula: Cl = [F • S (D/τ)]/Css, where F is the bioavailability fraction for the oral dosage form (F = 1 for most oral theophylline sustained-release products), S is the fraction of the theophylline salt form that is active theophylline (S = 1 for theophylline, S = 0.85 for anhydrous aminophylline, S = 0.80 for aminophylline dihydrate, S = 0.65 for oxtriphylline), D is the dose of theophylline salt in mg, Css is the steady-state theophylline concentration, and τ is the dosage interval in hours.
Occasionally, theophylline serum concentrations are obtained before and after an intravenous loading dose. Assuming a one-compartment model, the volume of distribution (V) is calculated using the following equation: V = (S • D)/(Cpostdose – Cpredose) where S is the fraction of the theophylline salt form that is active theophylline (S = 1 for theophylline, S = 0.85 for anhydrous aminophylline, S = 0.80 for aminophylline dihydrate), D is the dose of theophylline salt in mg, Cpostdose is the post-loading dose concentration in mg/L, and Cpredose is the concentration before the loading dose was administered in mg/L (both concentrations should be obtained within 30-60 minutes of dosage administration). If the predose concentration was also a steady-state concentration, theophylline clearance can also be computed. If both clearance (Cl) and volume of distribution (V) have been measured using these techniques, the half-life [t1/2 = (0.693 • V)/Cl] and elimination rate constant (k = 0.693/t1/2 = Cl/V) can be computed. The clearance, volume of distribution, elimination rate constant, and half-life measured using these techniques are the patient’s own, unique theophylline pharmacokinetic constants and can be used in one-compartment model equations to compute the required dose to achieve any desired serum concentration. Because this method also assumes linear pharmacokinetics, theophylline doses computed using the Pharmacokinetic Parameter method and the Linear Pharmacokinetic method should be identical.