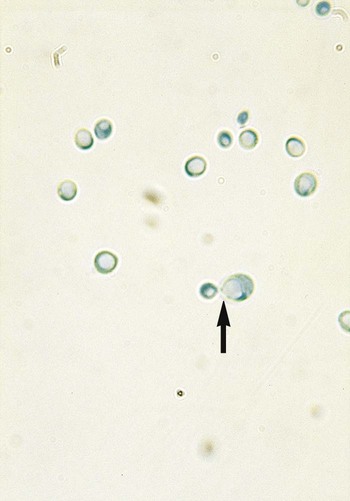
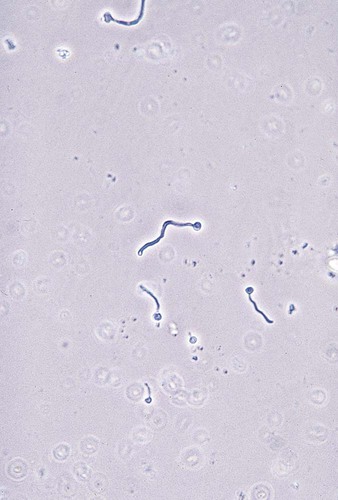
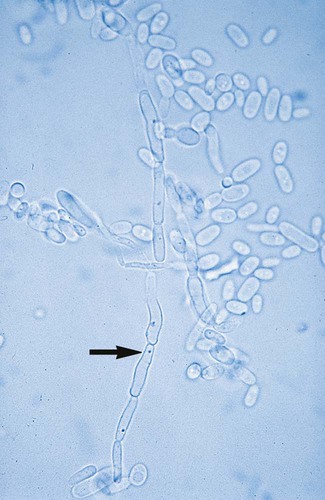
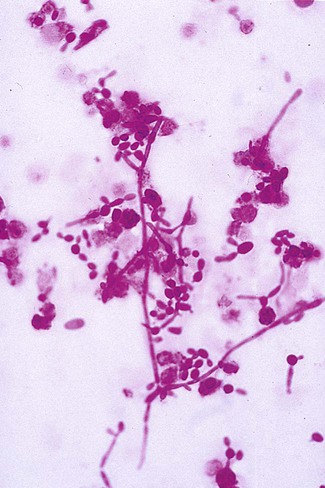
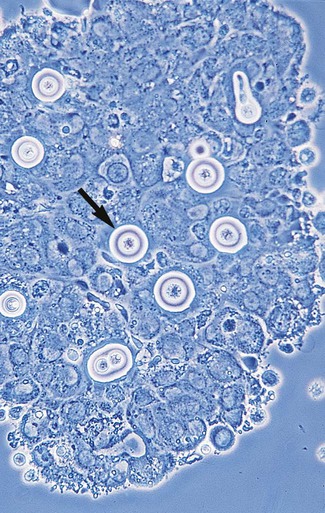
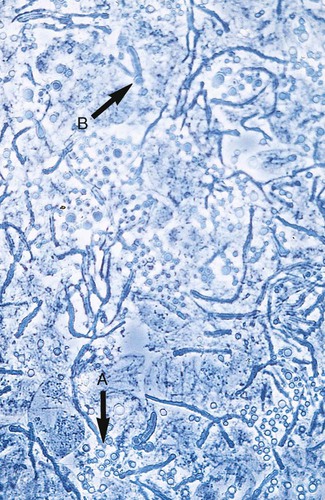
1. Explain the etiology and epidemiology of Candida, Cryptococcus, Trichosporon, and Malassezia spp. 2. Describe the macroscopic phenotypes and microscopic structures used to identify yeasts. 3. Differentiate yeast species based on microscopic, macroscopic, and biochemical test results. 4. Compare and contrast the methods used to identify yeasts, including staining, antigen testing, and biochemical and growth characteristics (i.e., morphology). 5. Diagram an algorithm for identifying the yeasts discussed in this chapter. In general, the yeasts reproduce asexually by blastoconidia formation (budding) (Figure 63-1) and sexually by the production of ascospores or basidiospores. The process of budding begins with a weakening and subsequent outpouching of the yeast cell wall. This process continues until the bud, or daughter cell, is completely formed. The cytoplasm of the bud is contiguous with the cytoplasm for the original cell. Finally, a cell wall septum is created between mother and daughter yeast cells. The daughter cell often eventually detaches from the mother cell, and a residual defect occurs at the budding site (i.e., a bud scar). With certain environmental stimuli, yeast can produce different morphologies. An outpouching of the cell wall that becomes tubular and does not have a constriction at its base is called a germ tube; it represents the initial stage of true hyphae formation (Figure 63-2). Alternatively, if buds elongate, fail to dissociate, and form subsequent buds, pseudohyphae are formed; to some, these resemble links of sausage (Figure 63-3). Pseudohyphae have cell wall constrictions rather than true intracellular septations delineating the fungal cell borders. Direct microscopic examination of clinical specimens containing Candida organisms reveals budding yeast cells (blastoconidia) 2 to 4 μm in diameter and/or pseudohyphae (Figure 63-4) showing regular points of constriction, resembling links of sausage. True septate hyphae (filamentation) may also be produced by C. albicans and C. dubliniensis. The blastoconidia, hyphae, and pseudohyphae are strongly gram positive. The approximate number of such forms should be reported, because the presence of large numbers in a fresh clinical specimen may have diagnostic significance. Microscopically C. glabrata blastoconidia are notably smaller (at 1 to 4 µm) than those of other medically significant Candida spp. Microscopic examination of other clinical specimens, including respiratory secretions, can be valuable for making a diagnosis of cryptococcosis. C. neoformans appears as a spherical, single or multiple budding, thick-walled yeast 2 to 15 μm in diameter. It usually is surrounded by a wide, refractile polysaccharide capsule (Figure 63-5). Perhaps the most important characteristic of C. neoformans is the extreme variation in the size of the yeast cells; this is unrelated to the amount of polysaccharide capsule present. It is important to remember that not all isolates of C. neoformans exhibit a discernible capsule. Microscopic examination of clinical specimens that contain Trichosporon spp. reveals hyaline hyphae, numerous round to rectangular arthroconidia, and occasionally a few blastoconidia. Usually hyphae and arthroconidia predominate. In white piedra, white nodules are removed and observed using the potassium hydroxide (KOH) preparation after light pressure is applied to the coverslip to crush the nodule. Hyaline hyphae 2 to 4 μm wide and arthroconidia are found in the preparation of the cementlike material that binds the hyphae together. The organism may be identified in culture by the presence of true hyphae, blastoconidia, and arthroconidia in conjunction with a positive urease (see Figure 60-40). Although Trichosporon asahii may be distinguished from other Trichosporon species by its biophysical profile (carbohydrate and substrate utilization), these organisms are likely best distinguished at the species level with molecular tools, such as DNA sequencing. M. furfur most often is detected through direct microscopic examination of skin scrapings. The organism is easily recognized as oval- or bottle-shaped cells that exhibit monopolar budding in the presence of a cell wall with a septum at the site of the bud scar. Small hyphal fragments also are observed (Figure 63-6); the morphology is commonly described as “spaghetti and meatballs.” In cases of fungemia, the morphologic form seen in direct examination of blood cultures is small yeasts without the presence of pseudohyphae.
The Yeasts
General Characteristics
Laboratory Diagnosis
Specimen Collection, Transport, and Processing
Stains
Candida spp.
Cryptococcus spp.
Trichosporon spp.
Malassezia spp.
The Yeasts