Fig. 30.1
Expression of PAX8 in normal and neoplastic renal tissues. PAX8 is expressed throughout renal tubules, but more intensely in distal tubules and collecting ducts (a, b), and urothelium lining the renal papillae (c). PAX8 expression tapers and patchy and weak expression are seen in urothelium lining the minor calyx (c). PAX8 expression is detected in the majority of renal cell neoplasms, including clear cell RCC (d, e), papillary RCC (f, g) and mucinous tubular and spindle cell carcinoma (h, i)
RCC Marker
RCC Marker (RCC Ma) is a monoclonal antibody raised against a glycoprotein on the brush border of proximal renal tubules. It is considered a “renal” marker as its expression is found in approximately 80 % of renal cell neoplasms, present in almost all low-grade clear cell and papillary RCC (PRCC) [9]. Its expression in other renal tumors is widely variable and the staining is often focal. It is absent in oncocytoma and collecting duct carcinoma. Its main disadvantage is the poor specificity with expression reported in many other nonrenal tumors , including neoplasms of parathyroid, salivary gland, breast, lung, colon, adrenal gland, testicular germ cell tumors, and mesothelioma. Its use to support the renal origin of a poorly differentiated tumor is now largely supplanted by other more sensitive and specific renal markers (i.e., PAX8 and PAX2).
CD10
CD10 is a cell-surface glycoprotein expressed on the proximal renal tubular epithelial cells and podocytes as well as many renal tumors with an expression pattern similar to that of RCC Ma. It has therefore been considered a useful marker to support the renal origin of a poorly differentiated neoplasm. Almost all clear cell and PRCCs are positive for this marker while other types of renal cell neoplasms are negative. Unfortunately, CD10 is even less specific than RCC Ma. Its expression is reported in wide array of nonrenal tumors, including carcinomas of lung, colon, ovary, and urinary bladder, and mesenchymal tumors such as endometrial stromal sarcoma and lymphomas. CD10 has fallen out of favor with the advent of PAX8/PAX2.
Human Kidney Injury Molecule-1 (hKIM-1)
hKIM-1 is a type I transmembrane glycoprotein expressed in injured proximal renal tubules. Its expression is also detected in the majority of clear cell and PRCC [10]. Only rare cases of chromophobe RCC and oncocytoma express this marker. It is therefore a relatively sensitive (80 %) and specific (90 %) marker for clear cell and PRCC, and metastatic RCCs. However, its expression is also detected in the majority (93.8 %) of ovarian clear cell carcinoma, 1/3 of endometrial clear cell carcinoma, and infrequently in colonic adenocarcinoma, limiting its use to narrow clinical circumstances.
Vimentin
Vimentin is found in the majority of RCCs. This stain alone is not a specific renal marker as its expression is found in wide range of neoplasms. Coexpression of vimentin and cytokeratin (CK), however, is limited to RCC and a few other carcinomas including endometrioid carcinoma, thyroid carcinoma, and mesothelioma. Therefore, coexpression of vimentin and CKs suggests RCC as one of the possible diagnoses.
Markers That Are Differentially Expressed in Different RCC Subtypes
Different histological subtypes of RCC are postulated to be derived from, or differentiate towards, different parts of nephron units which have distinct immunoprofiles. Therefore, renal tumors may be classified based on their immunoprofiles that recapitulate those of the normal nephrons. For example, CD10 and RCC Ma are found on the proximal renal tubules as well as in neoplasms that are derived from or recapitulate the proximal renal tubules (clear cell RCC and PRCC). Kidney-specific cadherin (ksp-cadherin), parvalbumin, claudins, and S100A are found on the distal nephrons and corresponding chromophobe RCC and oncocytomas. High-molecular-weight CKs are detected in collecting ducts of Bellini as well as in collecting duct carcinomas. However, morphology-immunophenotype concordance is imperfect. Such discordance occurs as the result of heterogeneity in tumor biology and technicality of immunohistochemistry. Furthermore, most published studies have utilized morphologically straightforward cases but not genetically confirmed difficult cases with ambiguous morphology. It should be emphasized that immunohistochemistry plays a supportive, rather than primary and definitive, role in the histological classification of RCC, and is best applied in the context of differential diagnosis.
Carbonic Anhydrase IX (CA9)
CA9 is a transmembrane protein of the carbonic anhydrase family. It is regulated by hypoxia inducible factor (HIF) and considered a marker for tissue hypoxia. CA9 is not expressed in healthy renal tissue as opposed to other carbonic anhydrase family members. It is instead expressed in most clear cell RCC (CCRCC) through HIF-1α accumulation driven by hypoxia or inactivation of the von Hippel–Lindau (VHL) gene [7, 11]. The staining pattern in CCRCC is circumferential membranous and is usually diffusely positive in most or all tumor cells (Fig. 30.2a). Focal staining is seen in up to one fourth of cases, typically in high-grade cancer (Fig. 30.2b). Its expression is also detected in clear cell tubulopapillary RCC (CCTPRCC), showing a unique “cup-like” pattern with staining decorating the basolateral, but not the apical, portion of cells lining glandular and cystic spaces (Fig. 30.2c). Its expression may also be detected in other high-grade tumors in the kidney including collecting duct carcinoma and pelvic UC (Fig. 30.2d), and can be seen adjacent to tumor necrosis due to ischemia and hypoxia (Fig. 30.2e, f). CA9 is not expressed in chromophobe RCC (ChRCC) and oncocytomas.
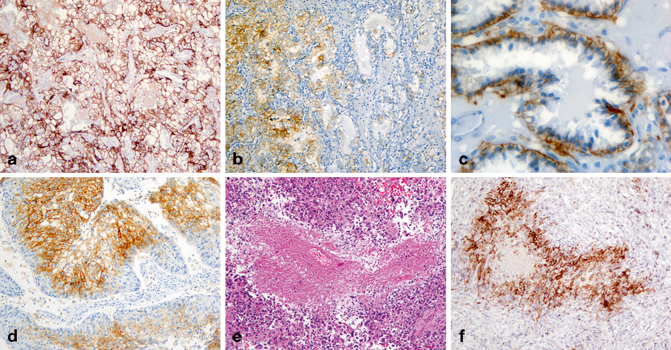

Fig. 30.2
Expression of carbonic anhydrase IX (CA9) in renal cell neoplasms. CA9 expression is diffuse and circumferential membranous in a clear cell RCC (a). The staining is focal in a high-grade clear cell RCC (b). In CCTPRCC, CA9 stains the basolateral, but not the apical, portion of tumor cells (“cup-like” pattern, c). CA9 is expressed in urothelial carcinoma (d). It is also expressed in cells surrounding necrosis (e, f) in an unclassified renal cell carcinoma
CA9 expression is also seen in many nonrenal tumors, including tumors of endometrium, stomach, cervix, breast, lung, liver, neuroendocrine tumors, mesotheliomas, and brain tumors. Therefore, CA9 has limited value in distinguishing renal versus nonrenal carcinomas. It is mainly used to confirm a diagnosis of CCRCC or CCTPRCC.
α-Methylacyl Coenzyme A Racemase
α-Methylacyl coenzyme A racemase (AMACR) is a mitochondrial enzyme involved in the oxidation of branched chain fatty acids and bile acid [12]. In the kidney, it is expressed in the proximal renal tubules (Fig. 30.3a). The majority of PRCC, both type 1 and 2, are positive for AMACR as granular cytoplasmic staining (Fig. 30.3b) [13]. Its expression is also found in mucinous tubular and spindle cell carcinoma, tubulocystic RCC, translocation RCC, but not in CCTPRCC, oncocytomas, and ChRCC. Therefore, positive AMACR staining provides support for a morphological diagnosis of PRCC.
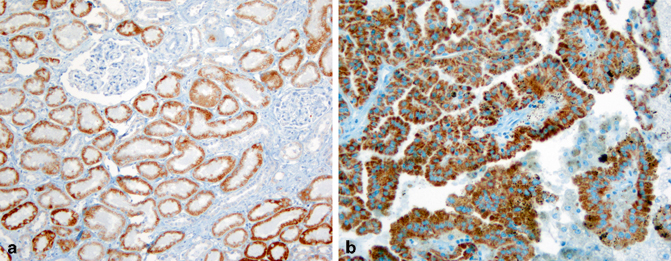

Fig. 30.3
Expression of α-methylacyl coA racemase (AMACR) in normal and neoplastic renal tissues. AMACR expression is detected in proximal renal tubules (a) and papillary renal cell carcinoma (b) as granular cytoplasmic staining
AMACR is found in a wide array of nonrenal tumors, most commonly in prostate adenocarcinoma, rendering it of little use in distinguishing renal from nonrenal tumors.
Parvalbumin
Parvalbumin is a calcium-binding protein involved in intracellular calcium homeostasis. In the kidney, its expression is limited to the distal nephrons from which ChRCC and oncocytomas are postulated to be derived. In support of such a histogenic derivation, parvalbumin expression is detected in these two subtypes of renal cell neoplasms, but is absent in other subtypes [14]. Therefore, parvalbumin immunostains may be used to differentiate oncocytoma and ChRCC from other renal tumors with similar “oncocytic” cytoplasm.
E-Cadherin and Kidney-Specific Cadherin
Epithelial cadherin (E-cadherin) is a calcium-dependent cell–cell adhesion glycoprotein. It is normally expressed in many cell types including renal tubular epithelial cells. Kidney-specific cadherin (ksp-cadherin) is an isoform of E-cadherin whose expression is exclusively found on the basolateral cell membranes of the distal convoluted tubules and collecting ducts (Fig. 30.4a, b) [15]. Both E-cadherin and ksp-cadherin are expressed in almost all ChRCC (Fig. 30.4c) and oncocytomas (Fig. 30.4d), but variably in other subtypes, including collecting duct carcinoma, translocation RCC, mucinous tubular and spindle cell carcinoma, and UC. Their expression in CCRCC and PRCC is, however, uncommon. Therefore, E-cadherin and ksp-cadherin may be used to distinguish ChRCC and oncocytoma from other renal tumors with “oncocytic cytoplasm.”
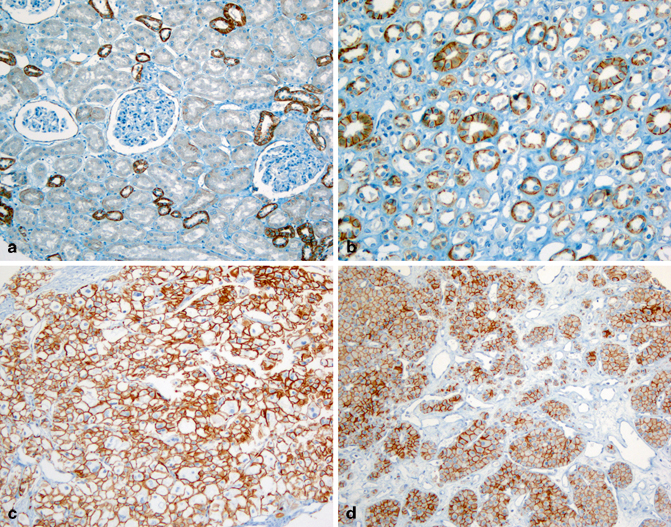

Fig. 30.4
Expression of epithelial cadherin (E-cadherin) in normal and neoplastic renal tissues. E-cadherin is only detected in a few distal convoluted tubules in the cortex (a), but is diffusely positive in the thick segments of loop of Henle and collecting ducts (b). Chromophobe RCC (c) and oncocytoma (d) are diffusely positive for E-cadherin
E-cadherin expression is commonly seen in other nonrenal tumors, often with positive staining in a high percentage of tumor cells, including lung, breast, and bladder carcinomas, rendering it unsuitable for differentiating renal from nonrenal tumors.
Claudin 7 and 8
Claudin 7 and 8 are members of a gene family that form tight cell junctions between epithelial cells. In the kidney, they are found primarily in the distal tubules and collecting ducts. Limited data show that claudin 7 and 8 are expressed in most ChRCC and oncocytomas, but in none or very few of other subtypes. Therefore, claudin 7 and 8 may be used in the differential diagnosis between ChRCC, oncocytomas, and other RCC with oncocytic cytoplasm.
CD117
CD117, or c-Kit, is a receptor tyrosine kinase that, upon binding to its ligands, phosphorylates and activates signal transduction molecules that propagate signals in cells and plays a critical role in cell survival, proliferation, and differentiation. Most ChRCC and oncocytomas are positive for CD117. However, no mutations have been identified in exons 9 and 11 of the c-Kit gene, the presence of which correspond to the therapeutic response to imatinib seen in gastrointestinal stromal tumors. Clear cell and PRCC are in general negative for CD117. Its expression has also been detected in sarcomatoid RCC and pelvic UCs.
S100A1
A member of S100 gene family, S100A1 is a calcium-binding protein whose expression is found in nephrons in the adult kidney. It is expressed in most oncocytomas, but in a significantly lower percentage of ChRCC cases. Such a differential expression pattern may aid in the distinction of these two tumors. Its expression, however, is also found in the majority of CCRCC and PRCC.
TFE3, TFEB, and Cathepsin K
Transcription factor E3 (TFE3) protein is encoded by the TFE3 gene on chromosome Xp11.2, and TFEB protein is encoded by the TFEB gene on chromosome 6p21. Both genes are members of the “microphthalmia transcription factor/transcription factor E (MiTF/TFE)” gene family. RCCs harboring chromosomal translocations involving the respective genes overexpress TFE3 and TFEB proteins which can be detected by immunohistochemistry [16–18]. Although molecular genetic analysis for the chromosomal translocation involving TFE3 and TFEB genes provides the most definitive evidence, immunohistochemical stains for TFE3 and TFEB proteins are sensitive, specific and highly correlate with the TFE3 and TFEB gene status in these tumors. TFE3 is undetectable in normal kidney tissues. TFE3 fusion protein, in contrast, is overexpressed in Xp11 translocation RCC (Fig. 30.5a, b) and is detected in over 95 % of Xp11.2 translocation RCC confirmed molecularly (Fig. 30.5c). However, TFE3 immunostaining can be seen in tumors other than Xp11.2 translocation RCC, including many perivascular epithelioid cell tumors (PEComa) of soft tissue and gynecological tract, a subset of which indeed harbors TFE3 gene alteration, as well as in a possibly related tumor, melanotic Xp11 translocation renal cancer. Rarely TFE3 immunoexpression is also detected in other tumors, including adrenal cortical carcinoma, granular cell tumor, bile duct carcinoma, and high-grade myxofibrosarcoma. The immunohistochemical stain for TFEB protein is both sensitive and specific for RCC associated with TFEB translocation, and is not detectable in other neoplasms. Weak nuclear staining for TFEB is rarely detected in scattered normal lymphocytes. The most significant issue with the immunohistochemical detection of TFE3 and TFEB proteins is that the staining is susceptible to tissue fixation. Inconsistent staining results are often encountered, especially when the staining is performed on an automatic stainer. Some staining protocols call for manual staining.
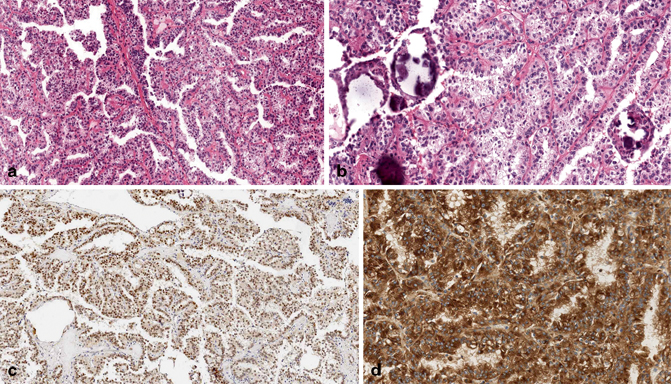

Fig. 30.5
a–d Expression of TFE3 and cathepsin K in a TFE3 translocation renal cell carcinoma (a, b). TFE3 staining is nuclear (c), while cathepsin K is cytoplasmic (d). (Courtesy of Dr. Guido Martignoni, University of Verona, Italy)
Cathepsin K is transcriptionally regulated by members of the MiTF/TFE gene family. Its overexpression is seen in all TFEB RCC (Fig. 30.5d) and 60 % of TFE3 RCC, but none of the other RCC subtypes [19, 20]. Its expression in nonrenal carcinomas is rare (2.7 %), although very common in mesenchymal tumors (> 50 %). These findings suggest that cathepsin K may be used as a surrogate marker for TFE3 and TFEB overexpression and is a highly specific marker for translocation RCC.
Markers for Urothelial Lineage Differentiation
Markers for urothelial lineage differentiation, including p63, thrombomodulin, uroplakin III, and GATA 3, are expressed in a high percentage of UC but not in RCC and therefore can be used in the diagnosis of a poorly differentiated carcinoma, where the differential diagnosis is between a UC and RCC [21]. One caveat is that p63 is reported to be expressed in small fraction of collecting duct carcinoma.
Cytokeratins
Different types of CK are expressed in different renal tumors and can be taken advantage of for the purpose of differential diagnosis. For example, CK18, a low molecular weight CK expressed in simple epithelia, is detected while CK20 is virtually absent in all major renal tumors. CK7, a low-molecular-weight CK, is expressed in PRCC (predominantly type 1), CCTPRCC, collecting duct RCC, and UC. High-molecular-weight CKs, detected by antibody clone 34βE12 and CK5/6, in contrast, are expressed in the majority of collecting duct RCC, almost all UCs and significant proportion of CCTPRCC, but uncommonly in other RCC subtypes.
Clinically several CK monoclonal antibody clones are used, including AE1/3, CAM5.2, 34βE12 and CK5/6. AE1/3 is considered a pancytokeratin as it detects both low molecular weight (CK7, 8, and 19) and high-molecular-weight (CK10, 14–16) CKs, but it lacks reactivity to CK18, a CK almost ubiquitously present in simple epithelia, including renal tumors. Notably AE1/3 is positive in only one third of CCRCC and one fourth of translocation RCC. If one wishes to confirm the carcinomatous nature of a poorly differentiated tumor in the kidney, a panel of markers, including AE1/3, CAM5.2, and CK18, should be used.
Immunophenotype of Common Renal Tumors
One has to bear in mind that characteristic immunoprofiles are derived from the studies of renal tumors of typical morphology . A poorly differentiated tumor often retains at least partially the characteristic immunoprofile of the renal tumors of the same histological class. However, significant deviation from the “typical” immunoprofile of a particular renal tumor type can occur and may impact the utility of these immunohistochemical markers in the classification of renal tumors. Therefore, while a concordant immunoprofile supports classifying the tumor under study into the subtype with that immunoprofile, a lack of concordance does not invalidate that classification.
Utility of Immunohistochemistry in Morphological Classification of Renal Tumors
With the exception of TFE3 and TFEB, none of the above-mentioned markers are specific for renal tumors. Immunostains should then be used to corroborate, rather than to establish, the morphological classification. One should always carefully examine the hematoxylin and eosin (H&E) morphology of the lesion first to generate a differential diagnosis and then apply appropriate markers. A panel of markers is preferred to include markers that support the favored diagnosis and markers that rule out other diagnoses included in the differential diagnosis.
Renal Tumors with Predominantly Clear Cell Nests and Sheets
Many renal tumors have clear, or pale-staining, cytoplasm as the predominant morphological feature (Table 30.1). Their characteristic morphological features should lead to the correct diagnosis, or at least narrow down the differential diagnosis in most cases. An initial panel of markers, including CK7, CA9, and ksp-cadherin (or CD117), can help when working up difficult cases. Additional markers can be performed judiciously based on the differential diagnosis. For example, urothelial markers, including p63, GATA-3, and high-molecular-weight CK (HMWCK) can be stained if UC is suspected. Adrenal cortical markers including inhibin and MelanA can be performed to rule out intrarenal adrenal cortical tissue.
Table 30.1
Histological features and immunoprofiles of renal tumors with “pale-staining” or clear cytoplasm
Tumor type | Morphological features | Immunohistochemical profiles | ||||
|---|---|---|---|---|---|---|
CK7 | CA9 | Ksp-cad | Cathepsin K | HMB45 | ||
CCRCC | Nests of clear cells of variable sizes divided by thin “chicken-wire” vascular septa; dilated sinusoid spaces | − | + | − | − | − |
CCTPRCC | Low grade clear cells forming long ribbons and lining papillae; nuclei polarized to the apical aspect | + | + (cup-like pattern) | − | −
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

| |