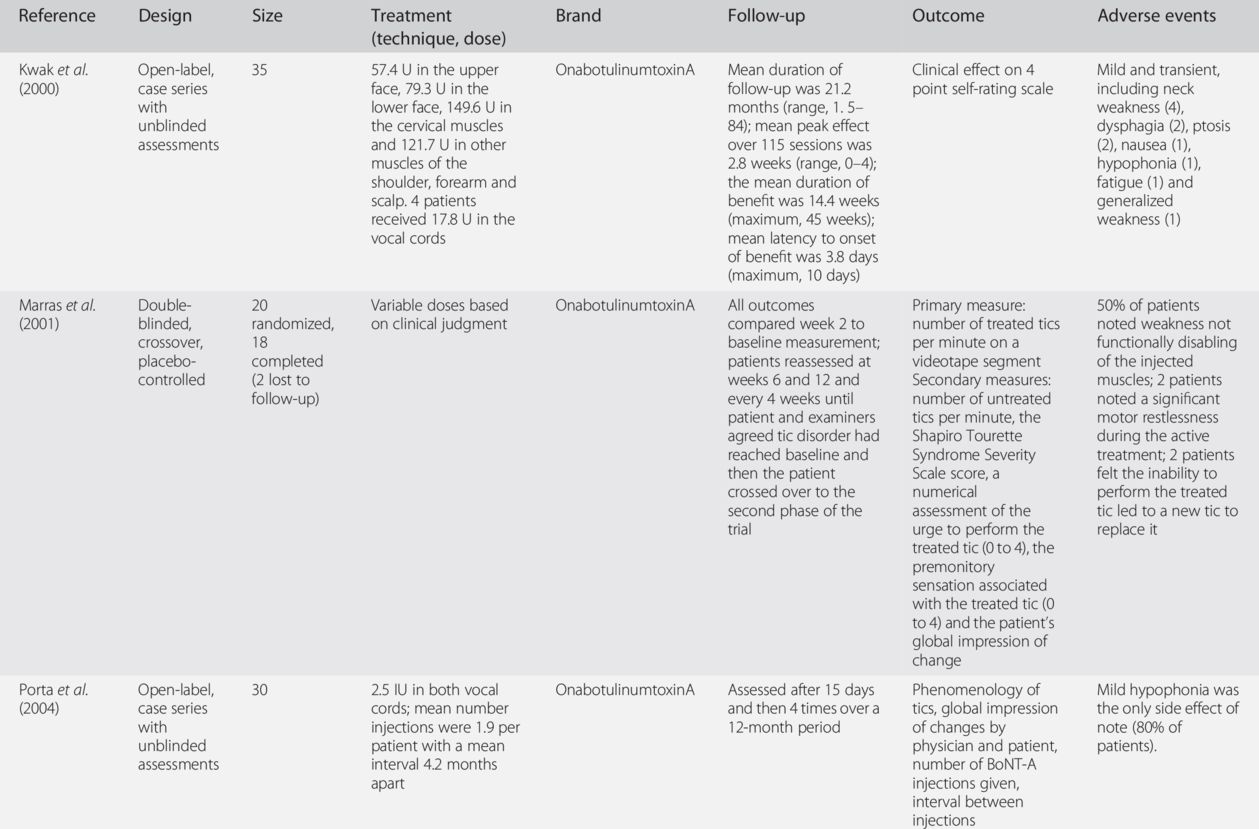
BoNT, botulinum neurotoxin.
Our experience with botulinum neurotoxin
Our long-term experience with BoNT in well over 1000 patients with tics provides further evidence that BoNT is a safe and effective treatment modality, particularly in patients with focal tics, such as blinking, facial grimacing, jaw clenching, neck extensions (“whiplash tics”) and shoulder shrugging.
Dosages and muscles injected
The exact muscles and location of injections are determined by considering which movements are of particular concern to the patient, by observing the predominant movement (including severity) of the tic being performed and by determining whether or not there is a significant localized premonitory sensation or urge associated with the tic. Dosing varies depending on the intensity of the premonitory sensation, force of the contraction and size of the muscle, but the average starting dose is 25–50 U onabotulinumtoxinA/incobotulinumtoxinA (onabotulinumtoxinA/incobotulinumtoxinA), 75–150 U abobotulinumtoxinA (Dysport) or 1500–2500 U rimabotulinumtoxinB (MyoBloc/NeuroBloc) into the splenius muscle (see Adult Dosing Guidelines and dosage recommendations of the WE MOVE Spasticity Study Group (2005)). The dosages of BoNT injected into vocal folds for phonic tics are, of course, substantially smaller, about 1–2 U onabotulinumtoxinA/incobotulinumtoxinA and 3–5 U abobotulinumtoxinA on each side. On occasion, as patients experience improvement of their treated tic, they may have a worsening of tics in other areas, but this is quite rare.
Tremors
Tremor is one of the most common movement disorders and essential tremor is the most common reason for referral to a movement disorders clinic for evaluation and treatment of tremor.
Clinical features
Essential tremor consists of involuntary, rhythmic or oscillatory movements, usually involving the hands, head and voice, and may be associated with other movement disorders such as dystonia and parkinsonism (Elble and Deuschl, 2011).
Treatment options for tremors
A recent review and practice parameter report by the American Academy of Neurology recommended propranolol, long-acting propranolol and primidone as the only first-line, class A medication therapies for essential tremor (Zesiewicz et al., 2011). Primidone is associated, however, with moderate to high frequency of acute adverse events and a decline in efficacy with long-term treatment in the majority of patients. Drugs such as topiramate, pregabalin and other anticonvulsants may also be useful in the treatment of essential tremor (Elble and Deuschl, 2011).
Use of botulinum neurotoxin
When oral medications for tremor have poor efficacy or intolerable side effects, BoNT injections may be used as an adjunctive treatment. There have been more than a dozen studies in which BoNT has been evaluated for efficacy and safety in treating hand tremor. The majority of these have focused on patients with essential tremor, but some have included subjects with Parkinson’s disease or parkinsonian rest tremor. There have been two randomized, double-blind, controlled studies to evaluate the efficacy of BoNT-A in treating essential hand tremor. In the first study by Jankovic et al. (1996), 25 patients were injected in both the wrist flexors and extensors with 50 U onabotulinumtoxinA and with an additional 100 U after 4 weeks if they failed to respond. Some of the patients had rest tremors, but all clinically met the criteria for essential tremor. Rest, postural and kinetic tremors were evaluated at intervals of 2 to 4 weeks for 16 weeks using tremor severity rating scales, accelerometry and assessments of tremor improvement and functional disability. A significant (p < 0.05) improvement on the tremor severity rating scale 4 weeks after injection was seen in the onabotulinumtoxinA-treated group compared with placebo. Additionally, at 4 weeks after injection, 75% of onabotulinumtoxinA-treated patients compared with 27% of placebo-treated patients (p < 0.05) demonstrated mild to moderate (peak effect of ≥ 2) subjective improvement in their tremor on a 0 to 4 rating scale. There were no significant improvements in the functional rating scales. Postural accelerometry measurements showed a ≥ 30% reduction in amplitude in 9 of 12 onabotulinumtoxinA-treated subjects and in 1 of 9 placebo-treated subjects. All patients treated with onabotulinumtoxinA reported some mild, transient degree of finger weakness.
In a randomized, multicenter, double-masked clinical trial by Brin et al. (2001), 133 patients with essential tremor were randomized to treatment with either low-dose (50 U) or high-dose (100 U) onabotulinumtoxinA or placebo. Injections were made into the wrist flexors and extensors and patients were followed for 16 weeks. Tremor severity was assessed with the hand at rest and in postural and kinetic positions. The effect of treatment was assessed by clinical rating scales, measures of motor tasks and functional disability, and global assessment of treatment. All assessments were scored on a scale of 0–4 measuring severity or disability (0, none; 1, mild; 2, moderate; 3, marked; 4, severe). Hand strength was evaluated by clinical rating and a dynamometer. The assessment of tremor severity based on rating scale evaluation indicated a significant difference (p < 0.05) from baseline for the low- and high-dose groups for postural tremor at 6, 12 and 16 weeks, and for kinetic tremor only at the 6-week evaluation, compared with placebo. Measures of motor tasks and functional disability were not consistently improved, but drawing a spiral and a straight line at 6 and at 16 weeks improved. The results of treatment on assessment using functional rating scales indicated that low-dose onabotulinumtoxinA significantly (p < 0.05) improved feeding, dressing and drinking at 6 weeks and writing at 16 weeks compared with placebo. In the high-dose group, onabotulinumtoxinA significantly (p < 0.05) improved feeding at 6 weeks; drinking at 6, 12 and 16 weeks; hygiene at 6 weeks; writing at 16 weeks; and fine movements at 6, 12 and 16 weeks. The Sickness Impact Profile scores and ratings on speaking, working, embarrassment and anxiety state were not significantly improved. The subjects had dose-dependent, finger or wrist weakness in flexion and extension, with a tendency for greater weakness in wrist and finger extension.
In both placebo-controlled studies, patients had statistically significant finger or wrist weakness in flexion and extension, with a tendency for greater weakness in wrist and finger extensors.
In an open-label study of BoNT treatment, 20 patients with disabling essential tremor not responding to conventional pharmacological therapy were enrolled (Pacchetti et al., 2000). Activities of daily living self-questionnaire, Severity Tremor Scale, accelerometry and surface electromyography were used to assess the severity of the tremor and identify the arm muscles involved in generating the tremor during certain positions. Treatment with BoNT was associated with a significant reduction in both severity and functional rating scales scores (activities of daily living self-questionnaire, Severity Tremor Scale) and of tremor amplitude as measured with accelerometry and electromyography (Table 18.2). The most common adverse effect, which occurred in 15% of patients, was a slight, transient, weakness of the third finger extension.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


