Fig. 26.1 Craniovascular innervation. CGRP, calcitonin gene-related peptide. (Reproduced with permission from Goadsby PJ (2001). Pathophysiology of headache. In Silberstein SD, Lipton RB, Dalessio DJ (eds.) Wolff’s Headache and Other Head Pain, 7th edn. New York: Oxford University Press; 2001, p. 59.)
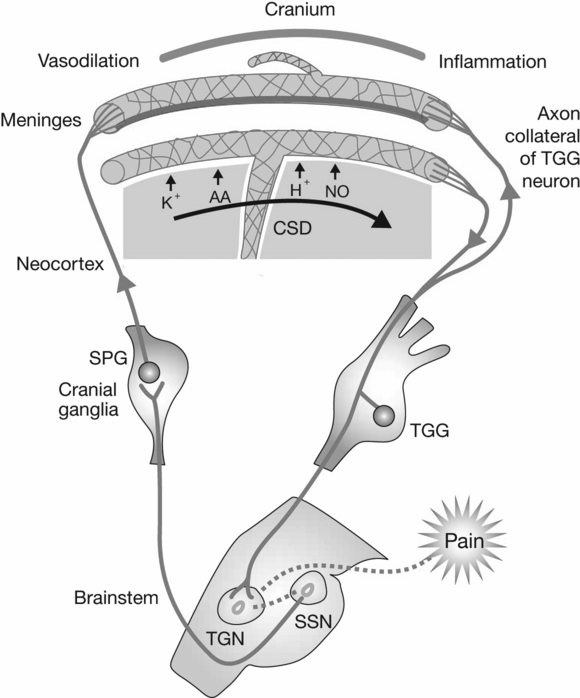
Fig. 26.2 Cortical spreading depression. AA, arachidonic acid; NO, nitric oxide; CSD, cortical spreading depression; TGG, trigeminal ganglion; SPG, sphenopalatine ganglion; TGN, trigeminal nucleus; SSN, superior sagittal sinus. (Reproduced with permission from Silberstein SD. Migraine. Lancet, 2004;363:381–91.)
The pathophysiology underlying TTH is not well understood. The relative contributions of peripheral and central pain mechanisms to TTH remain unclear (Silberstein et al., 2006).
Treatment of headache
Acute (abortive) migraine treatments, which patients take in an attempt to relieve pain and disability and prevent progression, include migraine-specific medications, such as ergots or triptans, and non-specific agents, such as analgesics or opioids (Silberstein, 2004). Patients with acute TTH typically self-medicate with over-the-counter analgesics, such as aspirin, acetaminophen, or non-steroidal anti-inflammatory drugs (NSAIDs), which could lead to drug overuse. They may also use prescription NSAIDs or combination analgesics.
Preventive treatments are designed to reduce the frequency, severity or duration of migraine attacks. These are indicated when acute medications are ineffective or overused or headaches are very frequent or disabling. Preventive agents include beta-adrenergic blockers, antidepressants, calcium channel and serotonin antagonists, anticonvulsants and NSAIDs (Silberstein, 2004). While daily oral-prophylactic treatments have proven effective, issues such as lack of compliance with daily dosing regimens and adverse effects have limited their usefulness (Blumenfeld et al., 2003; Silberstein, 2004) and this resulted in a search for other modalities and agents, including botulinum neurotoxin (BoNT), as potential preventive treatments.
Botulinum neurotoxin in headache disorders
Formulations
The seven BoNT serotypes (A1, A2, A3, B, C1, D, E, F and G) produced by Clostridium botulinum are synthesized as single-chain polypeptides. All serotypes inhibit acetylcholine release, although their intracellular target proteins, physiochemical characteristics and potencies are different (Aoki and Guyer, 2001; Mauskop, 2004). Serotype A (BoNT-A) has been the most widely studied serotype for therapeutic purposes (Aoki and Guyer, 2001). A major clinical advantage of BoNT-A arises from its extraordinarily prolonged duration of action due to the longevity of its protease (90 days in rats and probably much longer in human neurons). A di-leucine in the light chain of BoNT-A underlies its long duration of action by inhibiting its degradation (Wang et al., 2011).
Currently, BoNT-A is available for clinical use in the USA as onabotulinumtoxinA (Botox, Allergan, Irvine, CA, USA), abobotulinumtoxinA (Dysport, Ipsen, Slough, UK) and incobotulinumtoxinA (Xeomin, Merz Pharmaceuticals, Greensboro, NC, USA). Serotype B is available as rimabotulinumtoxinB (NeuroBloc/Myobloc, Solstice Neurosciences, South San Francisco, CA, USA). Lyophilized Botox is available in vials containing 100 or 200 U and is diluted with 2 or 4 ml of preservative-free 0.9% saline to yield a concentration of 5.0/0.1 ml or 2.5 U/0.1 ml, respectively (Allergan, 2004a). Reconstituted solutions of Botox can be refrigerated but must be used within 4 hours (Allergan, 2004a). Lyophilized incobotulinumtoxinA is available in vials containing 50 or 100 U and is diluted with preservative-free 0.9% saline. Dysport is reconstituted with 1.0 ml of 0.9% saline to yield a solution of 500 U/ml and the reconstituted product may be stored in a refrigerator for up to 8 hours before use (Allergan, 2004b). NeuroBloc/Myobloc is available in 0.5, 1 and 2 ml vials containing 5000 U/ml (Mauskop, 2004).
There is no formula for establishing dosage equivalence for onabotulinumtoxinA and abobotulinumtoxinA, which have different dosing, safety and efficacy properties. It has been difficult to establish dose conversion factors for BoNT-A preparations. In general, these formulations lack bioequivalence and interchangeability (Aoki and Guyer, 2001). In fact, the Botox package insert states that units of activity cannot be compared with nor converted into units of other BoNT preparations (Allergan, 2004a).
Mechanism of action of botulinum neurotoxin in headache
Botulinum neurotoxin binds to motor and sympathetic nerve terminals. It enters the nerve terminals and inhibits the release of acetylcholine. This inhibition occurs as the BoNT cleaves one of several proteins integral to the successful docking and release of acetylcholine from vesicles situated within nerve endings. This results in blocking of neuromuscular transmission at the neuromuscular junction. Following intramuscular injection, BoNT produces partial chemical denervation of the muscle, resulting in a localized reduction in muscle activity (Aoki and Guyer, 2001; Mauskop, 2004).
The association between BoNT use and the alleviation of migraine headache symptoms was discovered during initial clinical trials of BoNT-A treatment for hyperfunctional facial lines (Binder et al., 2000). Treatment with BoNT has been used for a variety of disorders associated with painful muscle spasms. Because migraine attacks are frequently associated with muscle tenderness (Jensen et al., 1998), it was generally believed that intramuscular BoNT might prevent abnormal sensory signals in the affected muscle from reaching the central nervous system. If abnormal muscle physiology can trigger migraine, one would predict that BoNT treatment would work prophylactically only in patients whose migraine attacks develop on the heels of episodic or chronic muscle tenderness.
Jakubowski et al. (2006) explored neurological markers that might distinguish patients with migraine who would benefit from BoNT treatment from those who would not. The prevalence of neck tenderness, aura, photophobia, phonophobia, osmophobia, nausea and throbbing was similar between responders and non-responders. However, the two groups offered different accounts of their pain. Among non-responders, 92% described a build up of pressure inside their head (exploding headache). Among responders, 74% perceived their head to be crushed, clamped or stubbed by external forces (imploding headache), and 13% attested to an eye-popping pain (ocular headache). The finding that exploding headache is not as responsive to extracranial BoNT injections is consistent with the view that migraine pain is mediated by intracranial innervation. The amenability of imploding and ocular headaches to BoNT treatment suggests that these types of migraine pain also involve extracranial innervation (Jakubowski et al., 2006). The precise mechanisms by which BoNT alleviates headache pain are unclear. Its inhibition of the release of glutamate and the neuropeptides substance P and calcitonin gene-related peptide from nociceptive neurons suggests that its antinociceptive properties are distinct from its neuromuscular activity (Dodick et al., 2005).
Botulinum neurotoxin may inhibit central sensitization of trigeminovascular neurons, which is believed to be key to migraine’s development and maintenance (Aoki, 2003; Cui et al., 2004; Oshinsky et al., 2004; Dodick et al., 2005). Oshinsky et al. (2004) used a preclinical model of sensitizing dorsal horn neurons in the trigeminal nucleus caudalis following chemical stimulation of the dura as a model for testing the effects of BoNT on central sensitization. They used single neuron electrophysiology of second sensory neurons in the trigeminal nucleus caudalis with cutaneous receptive fields and microdialysis of the trigeminal nucleus caudalis to evaluate the effects of pretreatment of the periorbital region of the rat with BoNT-A. In saline-treated animals, extracellular glutamate increased steadily after 100 minutes following the application of inflammatory soup to the dura. The increase in glutamate reached approximately three times the basal level at 3 hours after the inflammatory soup. Electrophysiologic recordings of neurons in the trigeminal nucleus caudalis before and after sensitization confirmed these data. Following the inflammatory soup, there was an increase in the magnitude of the response to sensory stimuli and an increase in the cutaneous receptive field of the second sensory neurons in the trigeminal nucleus caudalis.
Afferent–efferent communication happens in the nerve through axon–axon glutamate secretion, and at the level of the ganglion through non-synaptic release of glutamate and peptides (substance P and calcitonin gene-related peptide). Following a 5-minute chemical stimulation of the dura in a rat, the extracellular level of glutamate increases two- to three-fold. This increase was blocked by pretreating the face of the rat with BoNT-A. Producing the large changes in extracellular glutamate in the central nervous system requires a massive sensory activation. The afferents of the dura may recruit the afferents of the face and head, which leads to the sensitization of these areas seen in human and animal studies. Use of BoNT may block the axon–axon and interganglionic communication of the afferents and thus prevent central and peripheral sensitization outside of the dura. Electrophysiological studies confirmed that there is no change in the magnitude of the sensory response in the trigeminal nucleus caudalis neurons or their receptive field in the BoNT-A-treated rats following the inflammatory soup. These data show that peripheral application of BoNT-A prevents central sensitization elicited by stimulation of the dura with inflammatory mediators (Oshinsky et al., 2004).
Oshinsky et al. (2004) have proposed the following hypothesis: Following chemical stimulation of the dura (during a migraine attack and in this rat model), the dural afferents communicate with other trigeminal afferents on the ophthalmic division of the trigeminal nerve and recruit them to secrete glutamate and neuropeptides. This would recruit more afferents, spreading activation and sensitization. The number of afferents activated on the dura is small compared with the total number of afferents in the whole trigeminal system, so activation of the dural afferents alone may not be sufficient to produce the large changes in the central nervous system that lead to central sensitization.
Treatment guidelines
Therapy with BoNT-A is now indicated for patients with chronic migraine. It may also be effective for patients with a high frequency of episodic migraine. Its use is contraindicated for patients with sensitivity to BoNT. It must be used with caution in patients with neuromuscular disorders, such as myasthenia gravis (Blumenfeld et al., 2003).
Treatment techniques
Sterile technique should be observed for the entire BoNT injection procedure. Injections do not have to be intramuscular, but the muscles are used as reference sites for injections, which are most commonly administered in the glabellar and frontal regions, the temporalis muscle, the occipitalis muscle and the cervical paraspinal region.
The injection protocols commonly used are: (1) the fixed-site approach, which uses fixed, symmetrical injection sites and a range of predetermined doses; (2) the follow-the-pain approach, which often employs asymmetrical injections and adjusts the sites and doses depending on where the patient feels pain and where the examiner can elicit pain and tenderness on palpation of the muscle; and (3) a combination approach, which uses injections at fixed frontal sites supplemented with follow-the-pain injections (this approach typically uses higher doses of BoNT-A) (Blumenfeld et al., 2003).
Based on exploratory phase secondary chronic migraine studies (Castillo et al., 1998; Evers et al., 2004), the PREEMPT clinical program established a successful modified follow-the-pain protocol treatment paradigm (Elkind et al., 2006). OnabotulinumtoxinA (155 U) is administered as 31 fixed-site, fixed-dose injections across seven specific head and neck muscle areas. A sterile 1 ml Luer Lock syringe with a 30-gauge 0.5 inch (1.25 cm) needle is used. Each injection is 0.1 ml, which contains 5 U BoNT-A. Up to 40 U additional onabotulinumtoxinA can be administered, using a follow-the-pain strategy, into the temporalis, occipitalis and/or trapezius muscles, with a maximum dose of 195 U administered to 39 sites (Table 26.1). When deciding on dose and location of additional onabotulinumtoxinA, the location of the patient’s predominant pain and the severity of palpable muscle tenderness should be considered (Table 26.1 lists and Figs. 26.3–26.8 illustrate the recommended anatomical sites of injection for headache and the onabotulinumtoxinA (Botox) dose per site used in the PREEMPT trials).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


