Chapter 6
The Thoracic Region
This chapter first discusses the typical thoracic curve, vertebrae, ribs, and sternum. This is followed by a discussion of the thoracic vertebrae that have unique features (T1, T9 to T12). Next, ligaments with distinctive features in the thoracic region are covered. Many ligaments are described with the cervical region in Chapter 5 and are not covered again here. This chapter also includes a brief discussion of lateral curves that may develop in the thoracic region (scoliosis). The last section is devoted to nerves, vessels, and visceral structures associated with the thoracic vertebrae and the thoracic cage.
Thoracic Curve (Kyphosis)
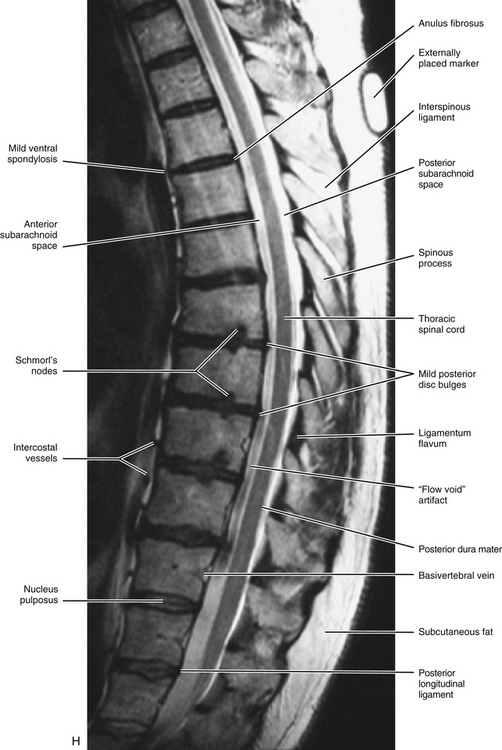
As stated in Chapter 2, the normal thoracic curve is a rather prominent kyphosis, which extends from T2 to T12 (Fig. 6-1, G, to 6-1, I). It is created by the larger superior-to-inferior dimensions of the posterior portion of the thoracic vertebrae (Masharawi et al., 2008).
Prolonged changes in the forces received by the thoracic region can result in postural changes and pain. For example, carrying book bags on one shoulder has been found to adversely affect posture and gait (Pascoe et al., 1997).
Occasionally the thoracic kyphosis is almost completely absent. This is logically called the straight back syndrome. This syndrome is associated with systolic heart murmurs and a distorted cardiac silhouette on x-ray film; as a result it can simulate organic heart disease. The straightening of the thoracic kyphosis results in a narrowing of the anterior-to-posterior dimension of the thoracic cage, which decreases the space available for the heart. The heart is forced to shift to the left, which leads to kinking of the great vessels. This results in a variety of heart murmurs. The straight back syndrome also has been associated with idiopathic mitral valve prolapse, a potentially life-threatening condition (Spapen et al., 1990).
Scheuermann’s Disease
Another clinical condition that can affect the thoracic kyphosis is Scheuermann’s disease. This disease is found in 0.4% to 8.3% of adolescents. Although its cause is unknown, there appears to be a genetic component to Scheuermann’s that is combined with increased physical stress and/or repeated trauma (Faingold et al., 2004). The condition has two forms based almost entirely on the location of the disease—classical Scheuermann’s disease, which affects the thoracic region and is seen in two thirds of cases; and Scheuermann’s disease of the lumbar region (one third of cases), which is generally considered to be the more painful form (Faingold et al., 2004). Both types usually occur during adolescence. Scheuermann’s disease is characterized by disruption of the cartilaginous end plate and fragmentation of the anular apophyses and the adjacent bone of the vertebral bodies (bony end plate) of many adjacent vertebral segments. The vertebral bodies become wedge-shaped (shorter anteriorly than posteriorly) if the disease is untreated. This results in an increase in the thoracic kyphosis, or in the case of the lumbar form of the disease, a continuation of the thoracic kyphosis into the upper lumbar region. As a result, the condition is the most common cause of a pathologic increase in the kyphosis of adolescents. Scheuermann’s disease affects males and females with equal frequency (Lemire et al., 1996).
Scheuermann’s disease usually begins at 10 to 12 years of age; however, radiologic changes are usually not apparent at this stage. The individual typically seeks treatment at 12 to 15 years of age because of aching back pain, an increase in thoracic kyphosis, or both. Irregularity and fragmentation of the bony end plates usually can be seen on x-rays at this time. The apex of the curve is at T8 in two thirds of the cases. The apex is usually in the upper lumbar region in the remainder of cases (lumbar type). Progression of the condition is slow in the beginning, increases as skeletal maturity of the spine occurs, and ends when vertebral growth is complete (up to 25 years of age). Multiple Schmorl’s nodes (end plate fractures) are another hallmark of the condition. Schmorl’s nodes typically are found in approximately 36% of all spines, but in up to 93% of spines with an increase in kyphosis resulting from Scheuermann’s disease. In addition, the intervertebral disc spaces usually narrow, especially in the segments affected by Schmorl’s nodes (Lemire et al., 1996).
In the classical form of Scheuermann’s disease, the affected individual may develop a stooped and round-shouldered appearance, with generally poor posture. Not all patients have pain; in those who do experience pain, it is frequently described as aching rather than constant and incapacitating. The pain almost always disappears as skeletal maturity is reached. The increased kyphosis that develops usually is rigid or fixed rather than flexible in nature (Lemire et al., 1996).
The severity of the condition typically is tracked by measuring the kyphosis and the vertebral wedging of affected vertebrae. The kyphosis is calculated by drawing lines anteriorly from the bony end plates at the superior and inferior boundaries of the kyphosis and then measuring the angle between these lines. The normal average is approximately 25 degrees (range, 10 to 40 degrees). A kyphosis greater than 45 degrees is indicative of Scheuermann’s disease. Vertebral wedging is measured by drawing a line anteriorly from the bony end plates of an individual vertebra. One or more vertebrae with wedging of greater than 5 degrees also is indicative of Scheuermann’s disease (Lemire et al., 1996).
Complications can occur in Scheuermann’s disease. The spinal cord may become compressed against the posterior surfaces of the vertebral bodies forming the peak of the kyphosis, leading to signs and symptoms of myelopathy (signs of a bilateral upper motor neuron lesion). In addition, in rare instances the condition may progress to form a significant deforming kyphosis (Lemire et al., 1996).
Treatment for Scheuermann’s disease includes bracing the spine in extension and providing palliative treatment for pain and discomfort. Exercise also has been found to be beneficial. Most patients respond well to such treatment (Lemire et al., 1996).
Typical Thoracic Vertebrae, Ribs, and Sternum
Vertebral Bodies
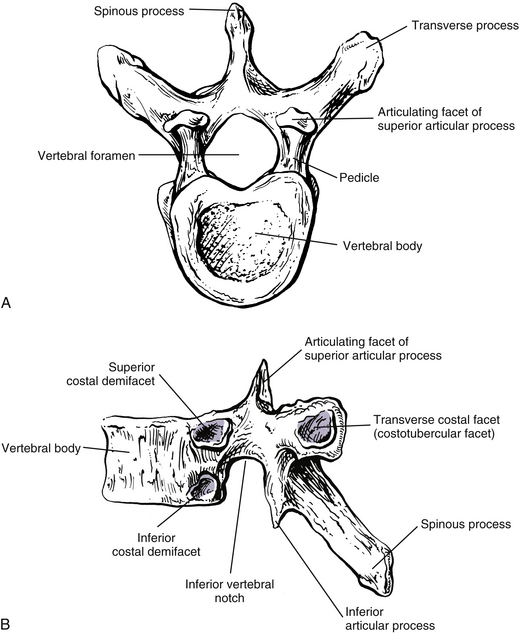
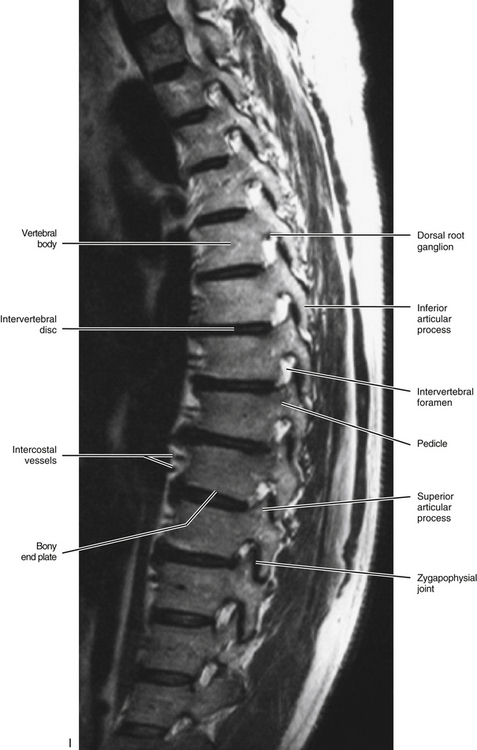
The size of the thoracic vertebral bodies is approximately the same in males and females of all races and of all adult ages (Masharawi et al., 2008; Limthongkul et al., 2010). The vertebral bodies of the typical thoracic vertebrae (T2 to T8) are larger than those of the cervical region (Fig. 6-1). The volume of the vertebral bodies increases from T1 to T12 (average 15.0 cm3; range 5.2 to 39.5 cm3) (Limthongkul et al., 2010). Thoracic vertebral bodies appear to be heart-shaped when viewed from above, primarily because of a marked concavity of the posterior aspect of the vertebral body in the region adjacent to the vertebral foramen. The posterior edge of the superior surface of upper thoracic vertebral bodies exhibits small remnants of the cervical uncinate processes (Dupuis et al., 1985).
The left-right (lateral) width of the thoracic vertebral bodies is greater than the anterior-posterior (length) and superior-inferior (height) dimensions. The lateral width decreases from T1 through T3 and then gradually increases throughout the thoracic region (continuing into the lumbar region until L4-L5). The decreasing width of the upper thoracic vertebral bodies may allow for increased lateral flexion (and possibly rotation) of the cervical region (Masharawi et al., 2008). In addition, the lateral width of a thoracic vertebra is smaller at the superior versus inferior vertebral margin of the vertebra and the lateral width of the inferior aspect of each vertebra is always larger than the superior width of the subjacent vertebra. Consequently, in a coronal (frontal) section the vertebral bodies have a trapezoid shape (i.e., narrower superiorly) and the intervertebral discs have an inverted trapezoid shape (i.e., narrower inferiorly) (Masharawi et al., 2008). Typical thoracic vertebrae also are more flattened on their left than right surfaces because of pressure from the thoracic aorta.
The thoracic vertebral bodies are wedged-shaped, the superior-inferior height being smaller anteriorly than posteriorly. The wedging increases from T1 to T7 and then begins to decrease incrementally until L2 (Masharawi et al., 2008). The posterior aspect of a typical thoracic vertebra is approximately five times the height of the posterior aspect of the intervertebral disc immediately above the vertebra (Harrison et al., 2003).
The superior-inferior height of most thoracic vertebral bodies is asymmetric with the right side usually greater than the left side. This “left lateral wedging” is typically found in one or several (usually 3) contiguous vertebrae, “interrupted” by a single vertebra of symmetric left and right heights, followed by additional vertebrae with left lateral wedging. This pattern is repeated throughout the thoracic region. Further investigation is needed to determine if the thoracic intervertebral discs normally compensate for the left lateral wedging of the vertebral bodies. The mild lateral wedging may help to provide stability to the thoracic region during the normal spinal loading (i.e., ground reaction force) that occurs throughout the day. The left lateral wedging is more pronounced in females (92% of females) than in males (86% of males). The female-predominant left lateral wedging may explain the clinical findings that the large thoracic curves of most adolescent idiopathic scolioses are most often found in girls and are usually convex to the right (Masharawi et al., 2008).
The superior-inferior height of the T1 and T2 vertebral bodies is greater than the anterior-posterior length. This ratio is reversed throughout the rest of the thoracic region. Consequently, the T1 and T2 vertebral bodies are more rounded when viewed from the left or right sides. This configuration may help to accommodate the large amount of flexion and extension that occurs in the cervical region (Masharawi et al., 2008). The T2 vertebral body is somewhat cervical in appearance, being slightly larger in transverse than anteroposterior diameter. The body of the T3 vertebra is the smallest of the thoracic region; the vertebral bodies gradually increase in size below this level. The vertebral bodies of T5 through T8 become more and more heart-shaped. This means that the concavity of the posterior aspect of the vertebral bodies becomes more prominent. The heart-shaped appearance also is accentuated because the anteroposterior dimension of the vertebral bodies increases more than the transverse (lateral) dimension at these levels.
The thoracic vertebral bodies become stronger from upper to lower thoracic vertebrae. This results from an increase in bone density that is probably a response to the increase in compressive forces placed on the successively lower vertebral bodies (Humzah & Soames, 1988).
The neurocentral synchondrosis (see Chapter 12), found between the neural arches and centra of developing vertebrae, fuses first in the lumbar and cervical regions and last in the thoracic region. This fusion may be incomplete in the thoracic region, even in adults (Edelson & Nathan, 1988).
Osteophytes are more prominent at T9-10 than at other thoracic levels. Generally, the aorta decreases the formation of osteophytes (bone spurs) on the thoracic vertebral bodies. Recall that the thoracic aorta courses along the left side of the thoracic vertebral bodies. It then begins to move to the anterior surface of the lower thoracic vertebrae, before passing through the aortic hiatus of the diaphragm. Consequently, osteophytes are found more on the right than left sides of the typical thoracic vertebral bodies (Nathan, 1962; Edelson & Nathan, 1988).
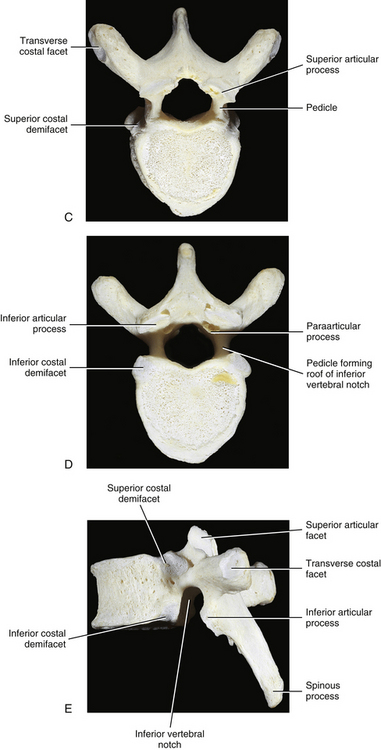
Typical thoracic vertebral bodies have four small facets, two on each side, for articulation with the heads of two adjacent ribs. These facets are known as costal demifacets (literally, “half-facets”) because the head of each rib articulates with both the superior demifacet of the vertebra with the same number and the inferior demifacet of the vertebra above (see Fig. 6-9). For example, the head of the sixth rib articulates with the superior demifacet of T6 and the inferior demifacet of T5. A ridge on the head of each rib, known as the crest of the head, is located between the two articular surfaces of the rib head. The crest of the head of each rib has a ligamentous attachment (intraarticular ligament) to the intervertebral disc (IVD) between adjacent thoracic vertebrae. A fibrous capsule surrounds each vertebral demifacet and continues to the rib surrounding the articular surface on the corresponding half of the rib head. The capsule is lined by synovium, making the costovertebral joint (costocorporeal joint) a synovial joint (diarthrosis). The radiate ligament extends from the head of each rib to the adjoining vertebral bodies and the surface of the intervening IVD (see Costocorporeal Articulations).
Several structures attach to the thoracic vertebral bodies. Table 6-1 summarizes these attachments.
Table 6-1
Attachments to Thoracic Vertebral Bodies
| Region | Structure(s) Attached |
| Anterior surface | Anterior longitudinal ligament, origin of longus colli muscle (T1, T2, T3, lateral to anterior longitudinal ligament) |
| Posterior surface | Posterior longitudinal ligament |
| Lateral surface | Origin of psoas major and minor muscles from T12 |
Pedicles
The pedicles of the thoracic spine are long and stout (Fig. 6-1). In fact, the pedicles in the lower thoracic region are larger than the pedicles of the upper lumbar vertebrae (Ofiram et al., 2007). The size of the thoracic pedicles varies considerably among both individuals and vertebrae, but the left and right pedicles of the same vertebra usually are similar (McLain, Ferrara, & Kabins, 2002). The T4 pedicles are the narrowest (left-to-right dimension) and then the pedicles of T5 to T12 become increasingly wider (Kretzer et al., 2011). The pedicles of males are wider than those of females (Pai et al., 2010; Kretzer et al., 2011), and there is ethnic variation in the size of the pedicles; for example, the dimensions of thoracic pedicles are smaller among East Indians and white populations (Acharya, Dorje, & Srivastava, 2010).
Unlike the pedicles of the cervical vertebrae, cancellous bone, rather than cortical bone, predominates in the thoracic pedicles. However, like the cervical pedicles, the cortical bone of the lateral wall of a typical thoracic pedicle is thinner than that of the medial wall (Kothe et al., 1996).
The thoracic pedicles become larger along their inferior surface from T1 to T12. Also, unlike the cervical pedicles, which attach at a significant lateral angle with the cervical vertebral bodies, the thoracic pedicles form only a slight lateral angle in the transverse plane with the thoracic vertebral bodies (and T12 forms no lateral angle with the vertebral body in this plane, i.e., a 90º angle to the vertebral body). The thoracic pedicles incline slightly superiorly in the sagittal plane (Marchesi et al., 1988). They also attach very high on their respective vertebral bodies; as a result, no superior vertebral notch is associated with typical thoracic vertebrae. T1, which is atypical, does have a superior vertebral notch (see First Thoracic Vertebra later in this chapter). On the other hand, the inferior vertebral notches of the typical thoracic vertebrae are very prominent.
Transverse Processes
The transverse processes (TPs) of typical thoracic vertebrae project obliquely posteriorly (see Chapter 2) (Fig. 6-1). They also lie in a more posterior plane than those of the cervical or lumbar regions, being located behind the pedicles, intervertebral foramina, and articular processes of the thoracic vertebrae (Standring et al., 2008). The TPs also become progressively shorter from T1 to T12; therefore the distance between the tips of the left and right TPs is the greatest at T1 and then decreases incrementally until T12, where the TPs are very small. This distance increases in the lumbar region (see Chapter 7). As with other components of thoracic vertebrae, the left and right transverse processes are asymmetric in length, with the left transverse process being longer than the right. In addition, the transverse process of males are larger than those of females (Masharawi & Salame, 2011).
The first six transverse costal facets are concave and face not only anteriorly but also slightly laterally. The transverse costal facets inferior to T6 are more planar (flatter) in shape and face anteriorly, laterally, and superiorly. The forces applied to the ribs during movements, load carrying, or muscular contraction are transmitted through the TPs to the laminae of the thoracic vertebrae (Pal et al., 1988).
The TPs serve as attachment sites for many muscles and ligaments. Table 6-2 lists the most important attachments to the TPs of thoracic vertebrae.
Table 6-2
Attachments to Thoracic Transverse Processes
| Region | Structure(s) Attached |
| Anterior surface | Costotransverse ligament (medial to transverse costal facet) |
| Apex | Lateral costotransverse ligament |
| Posterior apex | Levator costarum muscle |
| Inferior surface | Superior costotransverse ligament |
| Superior border | Intertransversarius muscle (or remnant) Intertransverse ligament |
| Inferior border | Intertransversarius muscle (or remnant) Intertransverse ligament |
| Posterior surface | Deep back muscles (longissimus thoracis, semispinalis thoracis and cervicis, multifidus thoracis, rotatores thoracis longus and brevis) |
From Standring S et al. (2008). Gray’s anatomy: the anatomical basis of clinical practice (40th ed.). Edinburgh: Churchill Livingstone.
Articular Processes
The superior articular processes of the thoracic spine are small superior projections of bone oriented in a plane that lies approximately 60 to 75 degrees to the horizontal plane (25 to 40 degrees to the coronal [frontal] plane) (White & Panjabi, 1990; Masharawi et al., 2004). This makes them much more vertically oriented than the cervical superior articular processes. The thoracic superior articular processes and their facets face posteriorly, slightly superiorly, and laterally (see Fig. 6-1). The left and right superior articular facets are usually asymmetric with the right facets being slightly more vertically oriented and the left ones facing slightly more laterally and also being slightly longer from superior to inferior (Masharawi et al., 2004, 2005). The inferior articular processes and their facets match those of the superior ones facing in the opposite direction—that is, anteriorly, slightly inferiorly, and medially, with matching asymmetry as well. The facet orientation and asymmetry are the same for males and females of all ages and racial origins (Masharawi et al., 2004).
The orientation of the thoracic articular processes and their articulating facets allows a significant amount of rotation to occur in this region (see Ranges of Motion in the Thoracic Spine). Flexion and extension are limited primarily by the orientation of the thoracic facets (Oda et al., 1996), and lateral flexion is limited partly by the orientation of the facets. However, the firm attachments of the thoracic vertebrae to the relatively immobile thoracic cage, by means of the costocorporeal and costotransverse articulations, are the primary constraints to axial rotation and lateral flexion of the thoracic spine.
Spondylolysis is a fracture of the region between the superior and inferior articular processes (pars interarticularis). It is usually found in the lumbar region (see Chapter 7 for a discussion of this condition), has been rarely found in the cervical region, and is considered extremely rare in the thoracic region. The first case reported in the English literature was that of Shimada and colleagues (2006), who reported a case of bilateral spondylolysis at T11 with forward slippage of the T11 vertebral body (spondylolisthesis).
Zygapophysial (Z) Joints
The zygapophysial (Z) joints are important structures clinically. These joints are thought to be the source of pain in 48% of the cases of chronic thoracic pain (Schulte et al., 2010). The capsules of the thoracic Z joints are similar to those of the cervical and lumbar regions. However, there are fewer mechanoreceptors in the Z joint capsules of the thoracic region than in the cervical or lumbar regions (McLain & Pickar, 1998).
Z Joint Synovial Folds (Meniscoids)
Z joint synovial folds (meniscoids) have been found to protrude into approximately 62% of thoracic Z joints, which is a lower incidence than the cervical and lumbar regions. Some thoracic Z joints have more than one synovial fold (Ley, 1974; Schulte et al., 2010). The folds are usually located in the inferior (caudal) aspect of the joints, although they have been identified in all regions of the joint. The folds are smaller in the thoracic region than in the other regions of the spine, but as in the cervical and lumbar regions, the thoracic synovial folds are believed capable of becoming entrapped (i.e., trapped between the articular facets) or extrapped (i.e., trapped between the articular process and Z joint capsule), both situations being a theoretical source of back pain (Kos, Hert, & Sevcik, 2002). In addition, Z joint synovial folds have been identified as a source of intraarticular bleeding associated with destruction of facetal cartilage (Schulte et al., 2010).
Thoracic Z joint synovial folds usually originate from a base of well-vascularized connective tissue and extend as flat, solid structures between the articular facets. The synovial lining is found at the base of these folds and usually does not extend between the facetal surfaces, which is different from the lumbar region where the synovial lining has been found to extend along the entire surfaces of the folds. The thoracic folds are longest in the lower thoracic Z joints, are of intermediate length in the upper thoracic region, and are shortest in the mid-thoracic region. These synovial fold lengths correspond to the mobility of the thoracic Z joints (most mobile to least mobile = lower, upper, and middle thoracic Z joints, respectively) (Schulte et al., 2010).
Approximately 6% of thoracic Z joint synovial folds are fatty in nature, also well vascularized, but are found exclusively in the joint recesses and do not extend between the articular facets. Occasionally, the adipose tissue found in the base of the solid (preceding paragraph) and fatty types of synovial folds passes through the ligamentum flavum and becomes continuous with the epidural adipose tissue of the vertebral canal (Schulte et al., 2010).
Another 4% of thoracic Z joint synovial folds are simply well-defined thickenings of the joint capsule (Schulte et al., 2010).
Laminae
The laminae in the thoracic region are short from medial to lateral, broad from superior to inferior, and thick from anterior to posterior. As with other components of thoracic vertebrae, the left and right laminae are asymmetric in superior-to-inferior length, with the right laminae being longer than the left. In addition, the laminae of males are larger (superior-to-inferior) than those of females (Masharawi & Salame, 2011). They completely protect the vertebral canal from behind. Therefore no space exists between the laminae of adjacent vertebrae in a dried preparation. This is unique to thoracic vertebrae. The rotatores longus and brevis muscles partially insert on the laminae of the thoracic vertebrae.
Paraarticular processes: Paraarticular processes are spurlike calcifications on the anterior and inferior aspects of the laminae of thoracic vertebrae (Figs. 6-1, D, and 6-6, B). They also have been called “laminar spurs” or “spicules,” and represent spur formations of the lateral-most attachment sites of the ligamentum flavum (LF) (Nathan, 1959). They are rarely found in the lumbar region, and they are almost never found in the cervical region. However, paraarticular processes are thought to be normal findings on thoracic vertebrae and are distinct from the condition known as ossification of the LF. Paraarticular processes have a relatively wide base and then taper to a dull point or blunt end inferiorly. They usually are paired and the left and right processes generally are of similar size, although they can be found unilaterally and also can be asymmetric in size. They are found throughout the thoracic region of the vertebral column, and their frequency increases as one descends the thoracic region from T1 to T10; the latter is the vertebra where they are most commonly found. The processes are also found at T11 and T12, but less frequently than at T10. Paraarticular processes occur with increasing frequency from 16 to 30 years of age, when they reach the maximum incidence; the incidence remains approximately the same throughout all succeeding age groups. The presence of paraarticular processes in younger age groups distinguishes them from osteophytes. The processes are thought to reinforce the attachment of the LF, adding strength to the attachment site. Nathan (1959) felt that their presence reflected increased strain placed on the LF as a result of the kyphotic architecture of the thoracic region.
More specifically, paraarticular processes attach to the lamina at the junction between the pedicle and inferior articular process and extend obliquely inferiorly and slightly anteriorly, forming a notch that opens inferiorly. The superior tip of the superior articular process of the vertebra below fits into this notch. Occasionally a bone spur extending from the superior articular process ascends and almost comes in contact with the paraarticular process. This latter process is a calcification of the inferior attachment of the LF. Other similar but smaller superior projections are found occasionally where the LF attaches along the superior margin of the laminae (Nathan, 1959).
The paraarticular processes vary in size from 1 to 15 mm in length, and the width of their attachment to the laminae ranges from 1 to 10 mm. They are found in 74.7% of spines, and are more common in whites than blacks or native Africans (whites, 82.1%; blacks, 63.8%; native Africans: Bantus, 62%; East Africans, 65%) (Nathan, 1959).
Occasionally paraarticular processes become quite large. These large processes could compress the exiting spinal nerve. However, a more likely scenario is that large paraarticular processes would predispose the exiting nerve to compression either from posterior osteophytes projecting from the vertebral body or possibly from IVD protrusion (Nathan, 1959).
Vertebral Canal
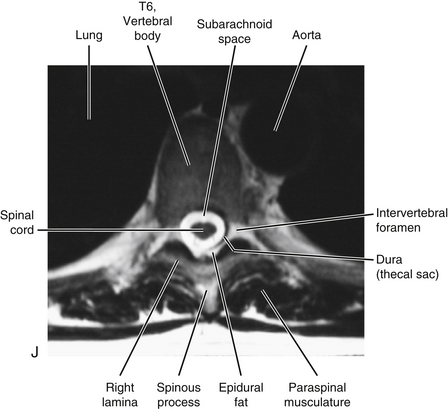
The vertebral canal in the thoracic region is more rounded in shape than in any other region (Fig. 6-1, A, C, and D). It is also smaller in the thoracic region than in either the cervical or the lumbar region (see Fig. 2-16). Compared to the other regions of the spinal cord, the thoracic spinal cord also is smaller.
Spinous Processes
The spinous processes of thoracic vertebrae generally are large (Fig. 6-1, B and E). The upper four thoracic spinous processes project almost directly posteriorly. The next four (T5 through T8) project dramatically inferiorly. The spinous process of T8 is the longest of this group. The last four thoracic spinous processes begin to acquire the characteristics of lumbar spinous processes by projecting more directly posteriorly and being larger in their superior-to-inferior dimension (see Unique Thoracic Vertebrae). The spinous processes of thoracic vertebrae serve as attachment sites for many muscles and ligaments. Because of the length of the thoracic region, attachments vary somewhat from the upper to lower thoracic vertebrae. Table 6-3 lists the attachments to the upper and lower thoracic spinous processes.
Table 6-3
Attachments to Thoracic Spinous Processes
| Region | Structure(s) Attached |
| Upper thoracic region | Ligaments: supraspinous, interspinous Muscles: trapezius, rhomboid major and minor, serratus posterior superior, deep back muscles (erector spinae and transversospinalis) |
| Lower thoracic region | Ligaments: supraspinous, interspinous Muscles: trapezius, latissimus dorsi, serratus posterior inferior, deep back muscles (erector spinae and transversospinalis) |
From Standring S et al. (2008). Gray’s anatomy: the anatomical basis of clinical practice (40th ed.). Edinburgh: Churchill Livingstone.
Intervertebral Foramina
The intervertebral foramina (IVFs) are covered in detail in Chapter 2. The IVFs in the thoracic region differ from those of the cervical region by facing directly laterally rather than obliquely anterolaterally. The lateral orientation of the thoracic IVFs is similar to that found in the lumbar region.
Unique to the thoracic region is that the T1 through T10 IVFs are associated with the ribs. The eleventh and twelfth ribs are not directly associated with IVFs. More precisely, the following structures are associated with the T1 through T10 IVFs: the head of the closest rib (e.g., T5-6 IVF associated with head of sixth rib), the articulation between the rib head and the demifacets of the vertebral bodies, including the associated ligamentous and capsular attachments with the vertebral bodies and the interposed IVD (see Costocorporeal Articulations). All these structures help to form the anterior and inferior boundaries of the first 10 thoracic IVFs. Pathologic conditions of these articulations may compromise the contents of the thoracic IVFs (Standring et al., 2008). For example, osteoarthritis (development of osteophytes) of the costocorporeal articulations may cause stenosis of the thoracic intervertebral foramina (Bailey & Casamajor, 1911).
Approximately one twelfth of the IVF contains a spinal nerve in the thoracic region, whereas up to 50% of the cross-sectional area of the IVF contains a spinal nerve (and dural root sleeve) in the cervical region (Sunderland, 1974), and approximately one third of the IVF is filled with a spinal nerve in the lumbar region. This may be one reason why radiculopathy as a result of IVD protrusion is much less common in the thoracic region than the lumbar or cervical areas. Thoracic disc protrusion is also less common than cervical or lumbar disc protrusion. One reason may be that the thoracic spine is rendered less movable than the cervical and lumbar regions. This is because the thoracic region is strongly supported by the ribs and sternum. The reduced motion may result in a reduction of stress on the thoracic IVDs.
Thoracic Cage
Discussing the bony elements of the thoracic cage here is appropriate because these elements are so intimately involved with the thoracic vertebrae. However, because the primary focus of this book is the spine, the ribs and sternum are not discussed in as much detail as the vertebrae. The intercostal muscles of the thoracic wall are covered in Chapter 4.
Components of the Thoracic Cage
The components of the thoracic cage (Fig. 6-2) include the following:
Superior Thoracic Aperture (Thoracic Inlet)
The term thoracic inlet has a slightly different meaning. It refers to the superior thoracic aperture, the region just above the first rib, and the opening between the clavicle and the first rib. Ironically the term thoracic outlet syndrome is frequently used to describe symptoms and signs arising from compromise of the neural or vascular structures as they pass through the region of the thoracic inlet. The symptoms associated with this syndrome typically are felt in the distal aspect of the upper extremity rather than the area of neurovascular compromise (Bland, 1987). The occurrence of thoracic outlet syndrome remains a matter of clinical debate, with some authorities stating that true compression of these structures is extremely rare. Others are convinced that such compression is relatively common. This section discusses the areas and structures typically associated with the thoracic outlet syndrome.
The right and left subclavian arteries and veins pass through the superior thoracic aperture. These vessels may be compromised by pathologic conditions of the lower cervical or upper thoracic viscera. Examples include lymphosarcoma affecting the lymphatics of the thoracic inlet (Moore, 1992) and tumors of the apex of the lung (Pancoast tumor), esophagus, and thyroid gland.
As the subclavian arteries and veins exit the superior thoracic aperture, they are met by the inferior structures of the brachial plexus. These neural structures include the anterior primary divisions of C8 and T1 and their union as the inferior trunk of the brachial plexus. All these vascular and neural structures pass over the first rib. The subclavian vein passes over the first rib in front of the anterior scalene muscle. The subclavian artery and lower (inferior) trunk of the brachial plexus course directly across the first rib between the insertions of the anterior and middle scalene muscles and are thought to be vulnerable in this region. Anomalous insertion of the scalenes or an anomalous inferior trunk of the brachial plexus that pierces either the anterior or the middle scalene muscles may provide the means by which these structures can become entrapped. Extension of the neck and rotation to the same side closes the interval between the anterior and middle scalene muscles, providing another possible mechanism of compromise. An elongated TP of C7 or a cervical rib (see Chapter 5) can dramatically crowd this region, and many believe that either one is a significant contributor to “thoracic outlet syndrome” (Bland, 1987; Foreman & Crofts, 1988). Cervical ribs range considerably in size, and even the smallest cervical rib can be associated with fibrous bands that course from the cervical rib to the first rib or sternum (van Es, Bollen, & van Heesewijk, 2010). Any or all of these structures could restrict the subclavian vessels and inferior trunk of the brachial plexus.
Terminology for Thoracic Outlet Syndrome
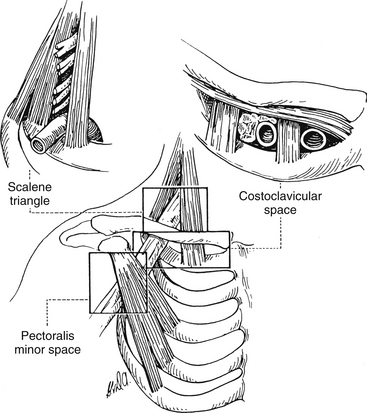
Thoracic outlet syndrome is characterized generally by numbness, paresthesias, pain, or a combination of these symptoms along the posterior aspect of the shoulder, axilla, and arm regions with or without vascular compression. However, there is much confusion related to the terminology surrounding thoracic outlet syndrome. Because of this confusion, Ranney (1996) has suggested a completely different nomenclature for the condition. Figure 6-3 illustrates the anatomic structures involved and the regions used in Ranney’s (1996) nomenclature.
Ranney (1996) first suggests that the term thoracic outlet syndrome be replaced with the term cervicoaxillary syndrome. Subdivisions of this syndrome would be named based on the specific anatomic region associated with the pathologic compression of neural or vascular tissues. Second, he suggests that the terms superior thoracic aperture and inferior thoracic aperture should be used instead of the clinically confusing terms thoracic inlet (for the superior thoracic aperture) and thoracic outlet (for the inferior thoracic aperture). Next, he proposes that the appropriate term scalene triangle be used for the space bordered by the anterior scalene muscle anteriorly, the middle scalene muscle posteriorly, and the first rib inferiorly. He then suggests that the superior aspect of this triangle, housing the C5, C6, and usually the C7 cervical nerve roots, be called the cervical outlet. (Compression of these structures in this region sometimes is called scalenus syndrome or scalenus anterior syndrome.) The term thoracic outlet would be reserved for the inferior aspect of the scalene triangle for the region that normally houses the C8 and T1 nerve roots and the subclavian artery. The subclavian artery or vein or the divisions or cords of the brachial plexus can be compressed in the more distal costoclavicular space. This space is bounded by the clavicle superiorly and first rib inferiorly. Finally, the pectoralis minor space is the region bounded superiorly by the pectoralis minor muscle and coracoid process to which it inserts, as well as the thoracic cage inferiorly. The term hyperabduction syndrome has been used to identify compression of the axillary artery and the cords of the brachial plexus against the pectoralis minor tendon during prolonged abduction of the upper extremity. With these terms and definitions in mind, Ranney (1996) suggests that the term cervicoaxillary syndrome (CAS) be used for compression of neural or vascular structures anywhere along the thoracic outlet, costoclavicular space, or pectoralis minor triangle, and that the more specific subtypes of CAS to include thoracic outlet, costoclavicular, and pectoralis minor syndromes be used once the precise location of compression is identified. The term cervical outlet syndrome would be used to describe compression of the C5, C6, or C7 nerve roots in the upper portion of the scalene triangle and the terms scalene syndrome and scalenus anticus syndrome would be discarded.