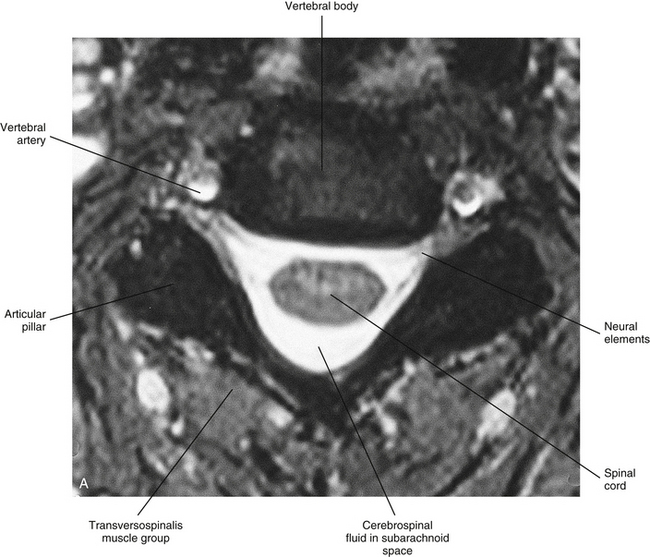
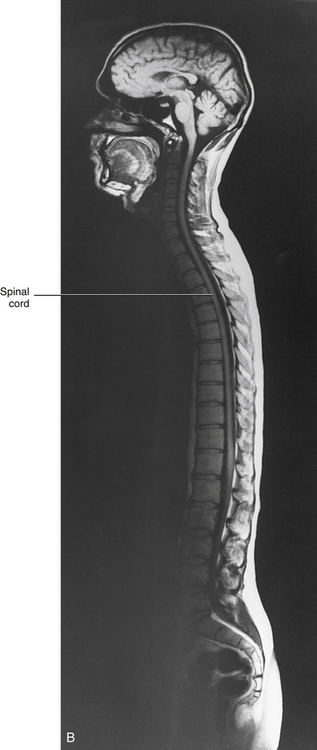
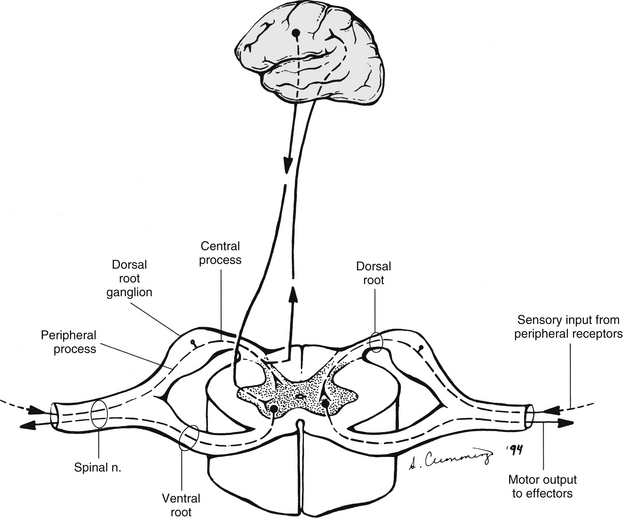
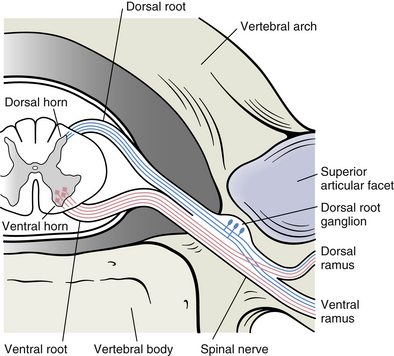
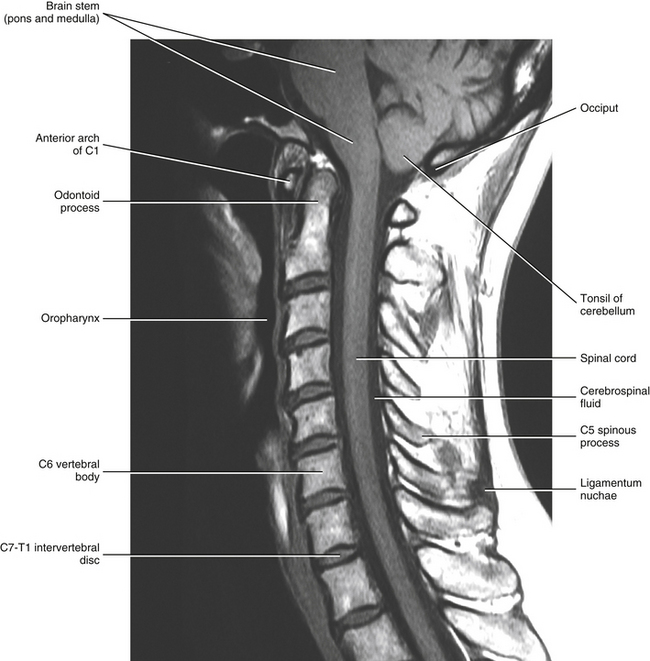
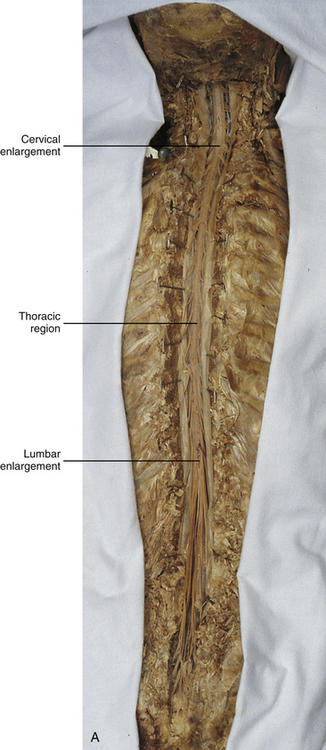
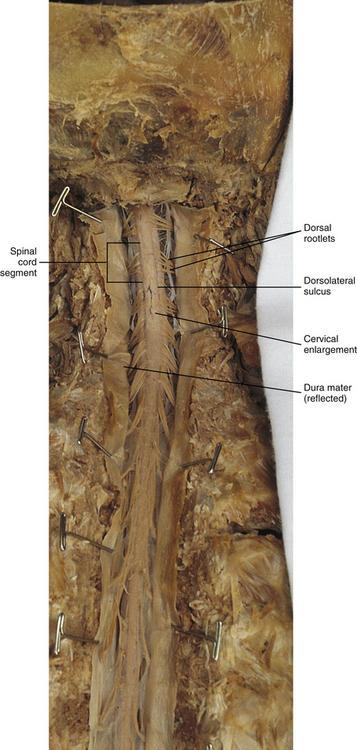
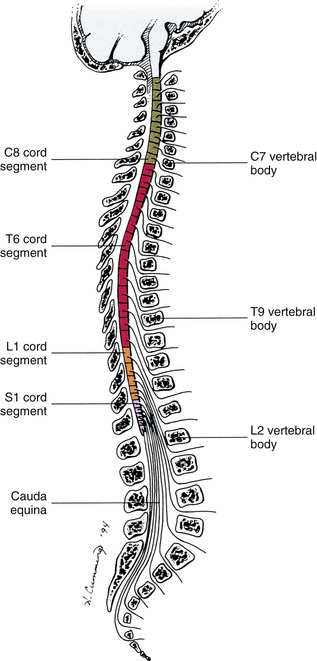
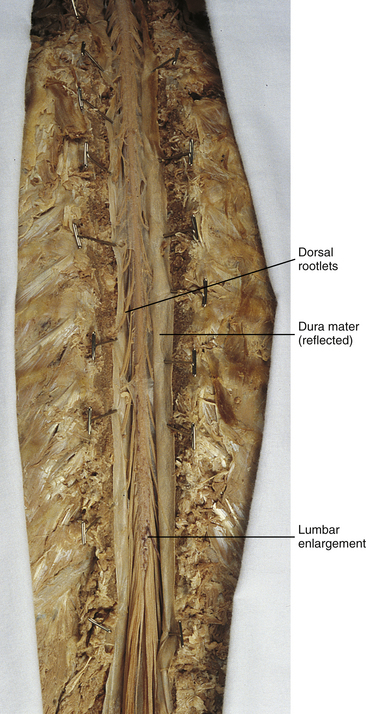
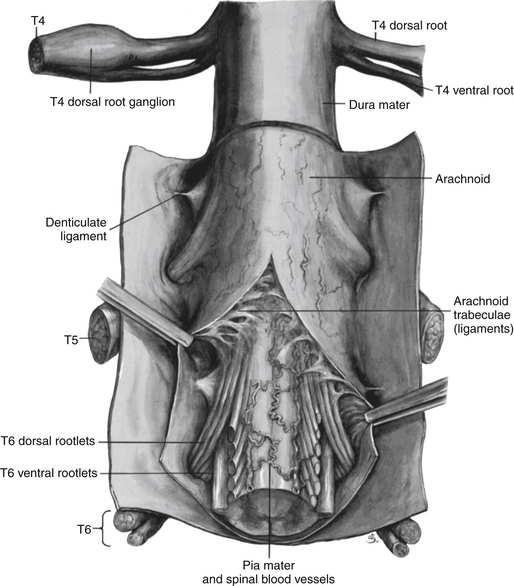
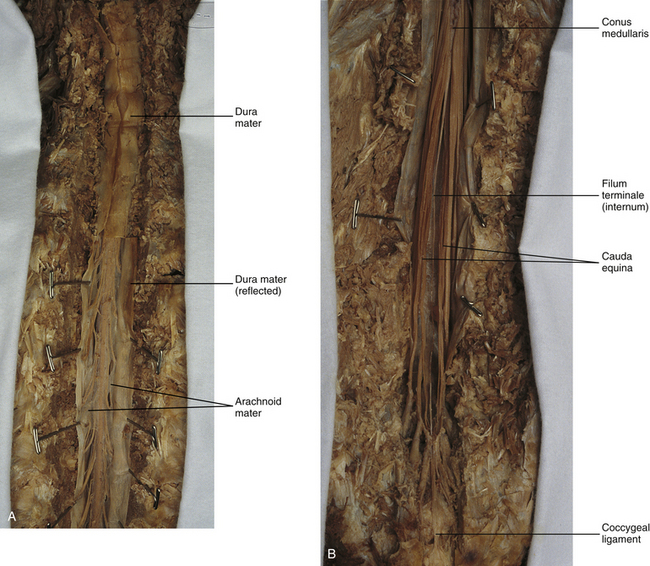
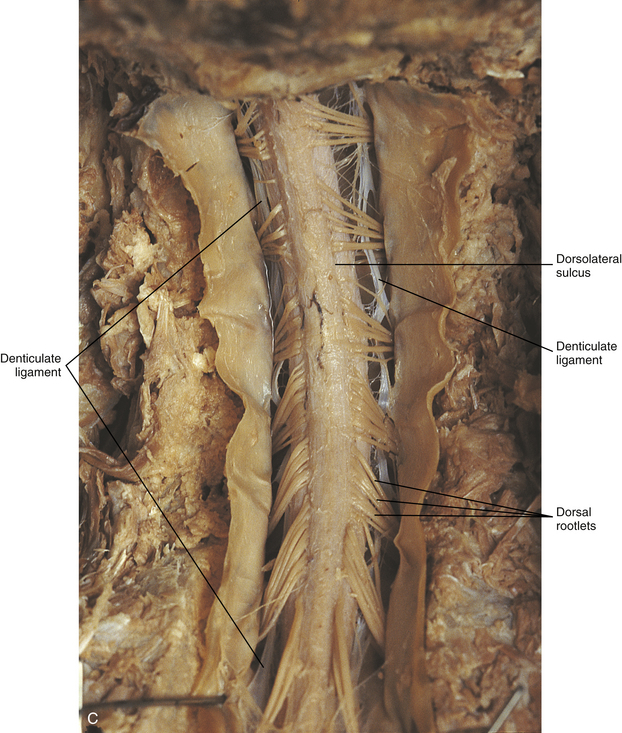
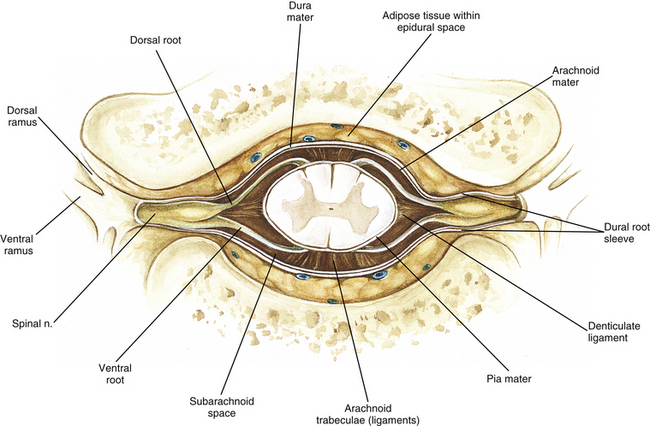
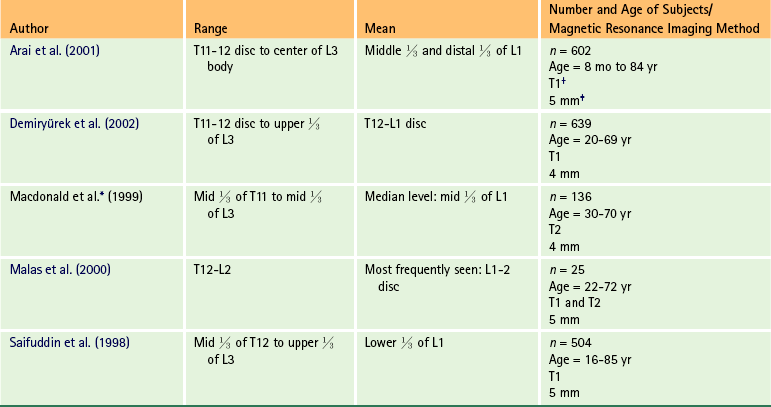
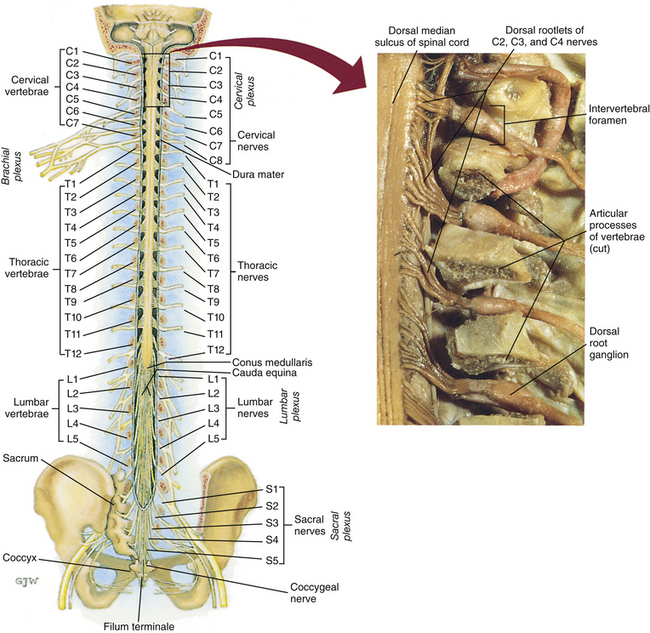
Chapter 3 The spinal cord, which is located in the vertebral (spinal) canal, is continuous cranially with the medulla oblongata of the brain stem and terminates caudally with a tapered inferior end. The cord is well protected by the vertebrae and the ligaments associated with the vertebrae. In addition to these bones and ligaments, cerebrospinal fluid (CSF) and a group of membranes, collectively called the meninges, also provide protection. The spinal cord does not lie immediately adjacent to the bone and ligaments but is separated from them by fluid, meninges, fat, and a venous plexus (Fig. 3-1, A and B). Within a peripheral nerve, the individual fibers are classified as part of either the somatic division of the nervous system or the visceral division of the nervous system. Somatic fibers (both sensory/afferent and motor/efferent) innervate skin, joints, tendons, and skeletal muscle located in the extremities and body wall. Visceral (or autonomic) fibers carry sensory/afferent information from the viscera to the CNS and provide motor/efferent control of smooth muscle, cardiac muscle, and glands. Twelve pairs of these peripheral nerves are associated with the brain (10 of which are attached to the brain stem) and innervate structures primarily in the head. These nerves, called cranial nerves, also convey special sense information such as hearing, vision, smell, and taste. In the context of this chapter, another group of peripheral nerves is more pertinent. These 31 pairs of nerves attach to the spinal cord; communicate with structures primarily located in the neck, trunk, and extremities; and are called the spinal nerves. In general, once input reaches the spinal cord via the spinal nerves, spinal cord neurons integrate and modulate the information and then send information back to the periphery as a motor response (i.e., reflexive, postural, or voluntary). In addition, such input to the spinal cord may result in the transmission of sensory input to higher brain centers for further processing. The higher centers then may transmit information down to the spinal cord neurons, which in turn relay it to the periphery, again via spinal nerves (Fig. 3-2). A spinal nerve is formed by the merger of two roots within the intervertebral foramen (IVF). One root, called the dorsal root, contains fibers that convey sensory information. The cell bodies of these sensory fibers are located in a reddish oval strucure called the dorsal root ganglion. The cell bodies are not found in the spinal cord because they developed from the neural crest (see Chapter 12). Each sensory neuron of the PNS is pseudounipolar because two processes diverge from one common stem (see Fig. 3-2). One of the processes is called the peripheral process and is continuous with a peripheral receptor. The other process is the central process, which courses in the dorsal root and enters the spinal cord (CNS). The dorsal root contains fibers of various diameters and conduction velocities that convey all types of sensory information. For example, some fibers in the dorsal root convey cutaneous sensory information from a specific area of skin called a dermatome. As the dorsal root approaches the spinal cord within the vertebral canal, it divides into approximately six to eight dorsal rootlets, or filaments. These rootlets attach in a vertical row to the cord’s dorsolateral sulcus. The other root, which helps form a spinal nerve, is called the ventral root and contains fibers that convey motor information to the body’s effectors, that is, all muscle tissue and glands. The cell bodies of these axons are located in the spinal cord. The axons emerge from the cord’s ventrolateral sulcus as ventral rootlets and unite to form one ventral root. Near the point of attachment of a dorsal or ventral rootlet to the spinal cord is the transition between the CNS and PNS. This CNS-PNS transition zone is a segment of the rootlet containing both CNS and PNS tissue. Each fiber coursing in a dorsal and ventral rootlet will cross a transition zone (TZ). Microscopically, the TZ consists of a peripheral PNS component overlying an axial CNS component. The TZ has a dome-shaped apex with a convex surface projecting in a distal direction. CNS fibers comprise the center of the dome, which is surrounded by an outer layer of astrocytes. The astrocytic processes project into the endoneurium of the peripheral nerve and intertwine with Schwann cells. Axons of the root fibers must pass through the network of astrocytic processes (Standring et al., 2008). Within the IVF the dorsal and ventral roots form the spinal nerve, which subsequently divides into its two major components: the dorsal and ventral rami (also known as the posterior primary division and anterior primary division, respectively) (Fig. 3-3). From this description, it can be seen that the spinal nerve and nerves formed distal to it (including the dorsal ramus and the ventral ramus) are mixed because they contain fibers conveying sensory input and fibers conveying motor output. However, proximal to the IVF, sensory and motor information is segregated in the fibers forming separate dorsal and ventral roots, respectively. This segregation of dorsal and ventral root fibers, and therefore of root function, was discussed and demonstrated by Bell and Magendie in the early 1800s and later was called the law of separation of function of spinal roots (law of Bell and Magendie) (Coggeshall, 1980). However, data do exist suggesting that the ventral roots of animals and humans contain some unmyelinated afferent fibers. It appears that the fibers (peripheral processes), the cell bodies of which are located in the dorsal root ganglion, travel into the ventral root and then loop back out and course toward the periphery (Coggeshall, Coulter, & Willis, 1973; Risling & Hildebrand, 1982; Risling et al., 1984; Azerad et al., 1986; Ko et al., 2009). At the level of the skull’s foramen magnum, the spinal cord becomes continuous with the medulla oblongata of the brain stem (Fig. 3-4). Although in cross section it is impossible to delineate the exact beginning of the cord and the end of the brain stem, grossly the beginning of the cord is easily distinguished by the definitive presence of the skull and vertebrae. As discussed in Chapter 12, a period during development occurs when the spinal cord extends the length of the vertebral column. However, while the vertebral column continues to develop in length, the spinal cord lags behind so that it eventually occupies the upper two thirds of the vertebral (spinal) canal (Fig. 3-1, B). It has been reported that at birth the cord ends at approximately the level of the L3 vertebra. Malas and colleagues (2000) used ultrasonography on full-term neonates (40 ± 2 weeks) and found the termination level of the cord to range anywhere from the L1-2 disc space to the L2-3 disc space. The spinal cord terminated at the L2-3 disc space in 50% (7 out of 14) of the neonates. In adults, because of continued greater growth of the vertebral column, the spinal cord ends approximately at the level of the L1 vertebra. In some individuals, however, the spinal cord may end as high as the disc between the T11 and T12 vertebrae or as low as the L3 vertebra (see Meninges and Table 3-1 for further description). At about the level of the caudal part of the T12 vertebral body, the cord tapers to a cone, which is known as the conus medullaris (Fig. 3-5, B). The overall length of the spinal cord to the inferior tip of the conus medullaris is approximately 42 cm in an average-sized woman and 45 cm in an average-sized man. The spinal cord’s weight is approximately 30 to 35 g. Remember that in most individuals the spinal cord does not extend inferior to the L2 vertebra. Therefore a lesion such as a herniated disc or trauma occurring below the L2 vertebra does not directly affect the spinal cord in most individuals. Table 3-1 Termination Level of the Conus Medullaris as Determined by Magnetic Resonance Imaging Scans ∗Macdonald and colleagues (1999) did not use the disc spaces as a possible point of termination. The intervertebral disc (IVD) was considered to be a part of the vertebral level above or below based on whether the conus ended at the upper or lower half of the disc (e.g., conus ending at upper part of the L1-2 IVD considered to end at L1 level). †T1 and T2, T1- and T2-weighted images, respectively. The external surface of the spinal cord is not a smooth surface but instead shows grooves of various depths called sulci and fissures. (When discussing cord anatomy, understand that the terms dorsal and ventral can be used interchangeably with posterior and anterior, respectively.) The spinal cord’s dorsal surface includes a midline dorsal median sulcus, right and left dorsal intermediate sulci (located from the midthoracic cord region superiorly), and right and left dorsolateral sulci. The cord’s ventral surface includes a midline ventral median fissure (approximately 3 mm deep) and right and left ventrolateral sulci. When inspecting the cord’s external surface, the dorsal and ventral rootlets are readily apparent, and the outward attachment to the cord of the paired (left and right) dorsal rootlets and paired ventral rootlets that serve one pair of spinal nerves defines one spinal cord segment (Fig. 3-6; see also Fig. 3-11, C). Therefore 1 pair of spinal nerves is associated with 1 cord segment; because 31 pairs of spinal nerves exist, there are also 31 spinal cord segments. These cord segments are numbered similarly to the numbering of the spinal nerves: 8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal cord segment. (Note that the first seven cervical nerves exit the IVF above their corresponding vertebra, and the remaining nerves exit below their corresponding vertebra. This allows one more cervical spinal nerve than cervical vertebrae.) Therefore the coccygeal segment is found at the very tip of the conus medullaris, which, as mentioned, is usually at the approximate level of the L1 vertebra (Fig. 3-7). This means that cord segments are not necessarily located at the same level as their corresponding vertebrae (Fig. 3-8). The relationship between cord segments and vertebral levels is always an approximation because cord length may vary among individuals. FIG. 3-7 Dorsal view of the external morphology of the spinal cord within the vertebral canal and the spinal nerves exiting within the intervertebral foramina. The tip of the cord (conus medullaris) is located approximately at the level of the L1 vertebra in this figure (see Table 3-1). The roots of the lumbar and sacral cord segments form the cauda equina, which is found in the lumbar cistern. The inset shows the cervical region, the dorsal rootlets, and the location of the dorsal root ganglia in the exposed intervertebral foramina. (From Thibodeau GA & Patton KT. [2003]. Anatomy & physiology [5th ed.]. St Louis: Mosby.) The spinous processes (SPs) of vertebrae serve as landmarks for identifying the approximate levels of cord segments; but in doing so the relationship of the vertebral body to the SP must be remembered. Therefore, in general, cervical SPs correspond to the succeeding cord segment, upper thoracic SPs correspond to cord segments two levels below, and lower thoracic SPs correspond to cord segments three levels below (Standring et al., 2008). For example, the C5 SP is at the level of the C6 cord segment; the T3 SP is at the level of the T5 cord segment; and the T10 SP is at the level of the L1 cord segment. The L1 through coccygeal cord segments, which include the segments responsible for the innervation of the lower extremities (i.e., L1-S3), are found at approximately the levels of the T10-L1 vertebral SPs. This anatomic relationship of cord segment to vertebra is important to remember for clinical reasons. For example, a patient with a fractured L1 vertebra does not experience the same lower extremity lesion signs and symptoms as a patient with a fractured T10 vertebra, because a T10 fracture typically injures upper lumbar segments and an L1 fracture injures the lower sacral and coccygeal segments. Although the spinal cord ends approximately at the L1 vertebra, each root that corresponds to a cord segment forms a spinal nerve and exits at its corresponding IVF. This includes the IVFs below the L2 vertebra. Therefore the rootlets and roots of the more inferior cord segments are longer and descend to their respective IVFs at a more oblique angle than the rootlets and roots of cervical segments, which are shorter and located almost at right angles to the spinal cord. The lumbosacral roots, therefore, become the longest and most oblique. The collection of these elongated lumbosacral roots coursing inferiorly to their corresponding IVFs is called the cauda equina (see Figs. 3-7, 3-8, and 3-11, B) because of its resemblance to a horse’s tail (the literal English translation of the term in Latin). In addition to the sulci and fissures, another anatomic characteristic seen on gross inspection of the spinal cord is the presence of two enlarged areas. One area is the cervical enlargement seen in cord segments C3 to T2. These cord segments include the segments that are responsible for the input from and output to the upper extremities. The other cord enlargement is the lumbar enlargement, which is visible from segments L1 to S3. These segments are responsible for the input from and output to the lower extremities. Because many more structures must be innervated in the extremities than the trunk, it is necessary to have more neuron cell bodies in the cord, and thus these two regions are enlarged (Standring et al., 2008) (Fig. 3-9; see also Figs. 3-5, A, and 3-6). Surrounding and providing protection and support to the spinal cord is a group of three membranes that are collectively called the meninges. The meninges surrounding the spinal cord are a continuation of the meninges surrounding the brain and consist of the dura mater (pachymeninx) and the leptomeninges, the latter being composed of the arachnoid mater and pia mater (Figs. 3-10, 3-11, and 3-12). During development, mesenchyme surrounding the neural tube thickens to form the primordial meninx. The outermost layer thickens and becomes the dura mater. The thin, innermost layer becomes infiltrated with neural crest cells and forms the leptomeninges. CSF fills spaces that coalesce (the future subarachnoid space) within, and subsequently separate the leptomeninges into two layers—the arachnoid mater and the pia mater (Moore & Persaud, 1998) (see the following text). As they separate, tiny strands remain and can still be identified in the adult subarachnoid space (Figs. 3-10 and 3-12). These strands are sometimes called arachnoid trabeculae or ligaments and are composed of a collagenous core surrounded by leptomeningeal cells (Standring et al., 2008). They may become concentrated and form septa in the vertebral subarachnoid space. The fully developed dura mater (pachymeninx) of the cord is the tough, outermost membrane and is a continuation of the inner layer or meningeal layer of dura mater surrounding the brain. It is separated from the vertebrae by the epidural space, which contains epidural fat, loose connective tissue, an extensive internal vertebral venous plexus, and fibrous bands called meningovertebral ligaments (see Chapter 2). The spinal dura mater attaches to the edge of the foramen magnum, the posterior aspect of the C2-3 vertebral bodies, and the posterior longitudinal ligaments (Standring et al., 2008) by fibrous bands of tissue. This elongated dural sac is held in place to the borders of the vertebral canal by the filum terminale externum and many other thickenings of connective tissue that vary in type in different regions of the vertebral canal (Barbaix et al., 1996; Fricke, Andres, & Von During, 2001; Dean & Mitchell, 2002; Humphreys et al., 2003). These connective tissue attachments to the dura mater are discussed in detail in Chapters 5 and 7. The recurrent meningeal nerve (or sinuvertebral nerve of von Luschka), which is formed outside the IVF and reenters the vertebral canal, provides significant innervation to the anterior aspect of the spinal dura mater. Although a few nerves sparsely innervate the posterolateral dura mater, the posteromedial region appears to have no innervation, which may explain why a patient feels no pain when the dura mater is pierced during a lumbar puncture (Groen, Baljet, & Drukker, 1988). The dura mater has been analyzed microscopically and has been found to be composed of outer and inner parts. The outer portion contains layers of fibroblasts, collagen fiber bundles (providing tensile strength and protection), and some elastic fibers. The latter afford flexibility for mechanical changes during movements and postural adjustments. The fibers are not arranged in a longitudinal or parallel fashion but are oriented in a variety of directions (Haines, Harkey, & Al-Mefty, 1993; Vandenabeele, Creemers, & Lambrichts, 1996; Reina et al., 1997, 1998; Fricke, Andres, & Von During, 2001). The inner portion of the dura mater lies adjacent to the arachnoid mater and consists of layers of cells called dural border, subdural, or neurothelial cells. These flattened cells are described as being sinuous with interdigitating processes that create extracellular spaces and few intercellular junctions (Haines, Harkey, & Al-Mefty, 1993; Vandenabeele, Creemers, & Lambrichts, 1996; Reina et al., 1998; Fricke, Andres, & Von During, 2001; Reina et al., 2002). Deep to the dura mater is the arachnoid mater, which is a vascular, delicate, and loosely arranged membrane (see Figs. 3-10 and 3-11, A). The outer portion consists of layers of flattened cells called arachnoid barrier cells. This multilayered structure is impermeable to CSF because it forms an anatomic and functional barrier between the dural blood supply and CSF in the subarachnoid space. Underneath the barrier cells is an extensive intermediate layer that is attached to the overlying arachnoid layer. This intermediate layer forms sheets that are highly perforated and have a lacelike appearance. Ultrastructurally, the intermediate layer is seen to consist of a core of collagen coated by leptomeningeal cells. This is similar to the structure of trabeculae in the cranial subarachnoid space. These sheets, which are most evident on the dorsal aspect, lie over the surface of the spinal cord. They laterally envelope spinal nerve roots and arteries and gradually disappear. This layer also develops into compact regions that form ligaments (also called trabeculae). Dorsal ligaments form a discontinuous series of connections from the outer arachnoid layer to the spinal cord, and dorsolateral ligaments extend from the outer layer to the dorsal roots. Although the ventral intermediate layer is less extensive, it forms ventral ligaments that have a similar arrangement to the dorsal ligaments. The intermediate layer may function as a baffle to help regulate CSF flow within the subarachnoid space. It may also be the site of considerable fibrosis from inflammation in the spinal subarachnoid space, leading to the complications of chronic arachnoiditis (Weller, 2005; Standring et al., 2008). Numerous studies (Haines, Harkey, & Al-Mefty, 1993; Vandenabeele, Creemers, & Lambrichts, 1996; Reina et al., 1998; Nolte, 2002; Reina et al., 2002) suggest that there is continuity between the inner surface of the dura mater and the arachnoid barrier cell layer such that there is no naturally occurring subdural space. Because the cellular characteristics of the dural border (neurothelial) cell layer create a structurally weak plane and an area of low resistance, disruption of this layer during surgery or by trauma, for example, can create an artificial subdural space. However, Hugh (2010) studied myelograms in which an oily contrast medium (Myodil) had been inadvertently injected into the subdural space instead of the subarachnoid space during lumbar puncture procedures. Based on the predictability, speed, and ease in which the oil traveled, Hugh suggested that a well-defined physiologic subdural space did exist. He speculated that the subdural space may be a lymphatic space or reservoir because it is connected to perineural lymphatic channels surrounding major peripheral nerves and to the central lymphatic system. The subdural space may also function as a major passageway in the reabsorption of CSF in humans when in an erect stance and also as a conduit for the spreading of metastatic tumors and pathologic organisms. Both the dura and the arachnoid (collectively forming the dural sac, or thecal sac) have typically been described as extending to the lower border of the S2 vertebra, well below the end of the spinal cord (conus medullaris). However, Macdonald and colleagues (1999) studied magnetic resonance imaging (MRI) scans of the lumbosacral region of 136 adults and found that the level of termination ranged from the upper border of S1 to the upper border of S4, with the median level being the middle one third of S2. They also noticed a gender difference in the termination level, with the median level for males being the upper one third of S2, and the middle one third of S2 the median level for females. This is supported by the work of Hansasuta and colleagues (1999). In addition to typically extending inferiorly to the S2 level, the dura and arachnoid also extend laterally and invest the nerve roots in a manner similar to a coat sleeve, as the roots travel distally toward the IVF to form their spinal nerve (see Fig. 3-12). At that point the dura blends in with the epineurial connective tissue surrounding the newly formed spinal nerve, whereas the arachnoid merges with the perineurium (Hewitt, 1970; Snell, 2001; FitzGerald & Folan-Curran, 2002; Standring et al., 2008). The subarachnoid space is under the arachnoid (see Fig. 3-12). This space is filled with CSF. At various locations throughout the CNS, the subarachnoid space becomes enlarged, forming regions known as cisterns. The subarachnoid space inferior to the conus medullaris is such an enlargement and is called the lumbar cistern (see Figs. 3-7 and 3-11, B). At this level the lumbar cistern contains not only CSF but also the cauda equina and filum terminale (see the following discussion). CSF is actively secreted, via various transport mechanisms, by the choroid plexus. The choroid plexus is specialized tissue located in the ventricles within the brain. Although CSF is often compared with plasma, its ionic composition is different; therefore CSF is not considered to be an ultrafiltrate of blood. CSF has numerous functions: it provides buoyancy and protection for the CNS against mechanical trauma; it provides a route for the removal of products of neuronal metabolism (sometimes referred to as “acting as a large metabolic sink”) (Benarroch et al., 1999; Nolte, 2002); it provides and regulates a stable chemical microenvironment to ensure normal functioning of neurons and glial cells; and it is a route by which neuroactive hormones may travel through the nervous system. Such neuroactive hormones include hypothalamic hormones that bind to distant target cells in the brain (Laterra & Goldstein, 2000) and pineal secretions traveling to the pituitary gland (Snell, 2001; Nolte, 2002). The CSF flows inferiorly in one direction within the ventricles located in the brain. At a level just rostral to the foramen magnum, most CSF leaves the most caudal (fourth) ventricle, enters the subarachnoid space, and flows superiorly over the brain and inferiorly around the spinal cord. In addition, a very small amount of CSF remains within the central canal of the cord. The CSF in the subarachnoid space gradually and slowly flows inferiorly into the lumbar cistern and then makes its way back superiorly. Pulsation of large spinal arteries within the subarachnoid space, respiratory movements, movements of the vertebral column, changing of body positions, and intrathoracic and intraabdominal pressure changes contribute to the flow of the CSF. Some CSF surrounding the cord flows superiorly and into the subarachnoid space surrounding the brain. Because of a pressure gradient, this CSF eventually flows from the subarachnoid space through arachnoid granulations (i.e., multiple villi that serve as one-way valves) and into the venous sinuses of the cranial dura mater. The CSF around the spinal cord also is absorbed through arachnoid villi that penetrate the dural root sleeves in the region of the dorsal root ganglia and project into small spinal veins leaving the IVF. It has been suggested that 25% of CSF reabsorption occurs through spinal arachnoid villi (Afifi & Bergman, 1998; Laterra & Goldstein, 2000; FitzGerald & Folan-Curran, 2002; Johnston & Papaiconomou, 2002; Nolte, 2002; Pollay, 2010). Emptying into the venous system completes the one-way circulation of the CSF. Human and animal studies also suggest that CSF drains into lymphatic vessels and subsequently into regional lymph nodes (Boulton et al., 1996; Miura, Kato, & von Ludinghausen, 1998; Fricke, Andres, & Von During, 2001; Snell, 2001; Johnston & Papaiconomou, 2002; Pollay, 2010). The innermost membrane of the meninges is called the pia mater. This layer consists of one to two layers of flattened cells that are joined by desmosomes and gap junctions. They are continuous with the leptomeningeal cells of the ligaments of the arachnoid intermediate layer. Separating this layer from the neural tissue of the external surface of the spinal cord (the astrocytic glia limitans) is the subpial space. This space contains a substantial amount of collagen fiber bundles, fibroblast-like cells, and blood vessels, such as the anterior spinal artery. The pia mater invests the spinal cord and forms a fold within the ventral median fissure. It also surrounds the arteries, rootlets, and roots as they course into the IVF. In addition to acting as a separation between the subarachnoid and subpial spaces, it has been suggested that the pia mater surrounding the brain may serve as a regulatory interface between the two spaces by means of the pinocytotic action of the pial cells (Standring et al., 2008). Two specializations of pia mater that function to stabilize the spinal cord within the vertebral canal are the left and right denticulate ligaments and filum terminale. Each denticulate ligament is a collagenous, triangular-shaped serrated ribbon coursing the length of the cord. It consists of a medial border that is continuous with the subpial connective tissue of the spinal cord, and a lateral apical border that attaches to the dura mater in an even distribution at approximately 21 points on each side along the cord’s length. Because of its location, the denticulate ligament forms a shelf within the vertebral canal between the dorsal and ventral roots (see Figs. 3-10, 3-11, C, and 3-12) and divides the canal into anterior and posterior compartments. Superiorly the ligament attaches to the dura mater above the lateral rim of the foramen magnum and behind the hypoglossal nerve. The ligament lies between the anteriorly placed vertebral artery (which separates the ligament from the first cervical ventral root) and the ascending spinal root of the spinal accessory nerve. The most inferior portion arises from the conus medullaris and descends as a narrow oblique band to attach laterally to the dura mater lying between the roots of the exiting T12 and L1 spinal nerves. Cineradiography has shown that the form and position of the denticulate ligaments may change during movements of the spine (Standring et al., 2008). In the cervical region, the lateral apical attachments of the left and right denticulate ligaments attach to the dura further away from the exiting nerve roots than those of the lower thoracic region (Kershner & Binhammer, 2002). Tubbs and colleagues (2001) dissected 12 cadavers and described the morphology of the ligament in detail. They described the lateral apices of most ligaments as consisting of superior and inferior prongs that were approximately 1 mm in length. These two pronged ligaments were most prominent in the cervical and upper thoracic regions; the lower thoracic ligaments were not pronged. The lateral apical attachments in the cervical region were found to be thicker than those found in the thoracic and lumbar regions. Based on gross inspection and electron microscopy of 56 cadavers, Kershner and Binhammer (2002) suggested that developmental remnants of the lateral apex of denticulate ligaments formed “intrathecal ligaments” that were associated with the cauda equina. An average of 18 such ligaments per cadaver were found within the lumbar cistern. These dense collagenous intrathecal ligaments were covered with a thin layer of leptomeningeal cells and varied in thickness (0.13 to 0.35 µm) and length (3 mm to 3.5 cm). Some of the ligaments randomly connected dorsal nerve roots of the cauda equina to the dura mater, and occasionally the ligaments joined dorsal and ventral roots. These intrathecal ligaments are thought to be derived from the denticulate ligaments because the spinal cord ascends relative to the rest of the vertebral column during development. The clinical significance of these structures is unknown. The other special component of pia mater is a bluish-white structure called the filum terminale. This slender filament consists of glial cells and ependyma (ependymal cells line the central canal of the spinal cord), and is covered by pia mater. The first 5 to 6 mm of the filum terminale includes a central canal. The filum extends approximately 20 cm from the tip of the conus medullaris within the lumbar cistern to the dorsum of the coccyx, where it blends into the connective tissue covering this bone (see Fig. 3-11, B). The portion of the filum terminale between the conus medullaris and the inferior tip of the dural sac is known as the filum terminale internum (approximately 15 cm long). Because the filum terminale pierces the dura and arachnoid at the S2 level on its way to the dorsum of the coccyx (thereby exiting the lumbar cistern), the filum terminale picks up two additional layers (dura and arachnoid); thus from S2 to the coccyx, it is usually called the coccygeal ligament (filum terminale externum). A few strands of nerve fibers adhere to the upper part of the filum. It has been suggested that these are likely to be rudimentary roots of the second and third coccygeal spinal nerves (Standring et al., 2008). The filum terminale has been described as a viscoelastic band that functions to “fixate, stabilize, and buffer the distal cord from normal and abnormal cephalic and caudal traction” (Bui, Tubbs, & Oakes, 2007) and to allow minimal movement of the conus medullaris during flexion and extension of the spine. The filum terminale may seem to be an unassuming remnant but it does have clinical significance in its contribution to a condition known as tethered cord syndrome (TCS). Originally the term “tethered spinal cord” described patients with an abnormally low conus medullaris that was “tethered or anchored” by a thickened (≥2 mm in diameter) filum. The definition of a tethered cord has expanded and is now associated with many disorders including a wide range of occult spinal dysraphisms (OSDs), trauma, infection, and neoplasm (Bui, Tubbs, & Oakes, 2007). TCS can be seen in children because of a congenital closed neural tube defect. In this developmental situation the filum terminale becomes abnormally thick or infiltrated with fat and its viscoelasticity is lost or diminished. This places tension, traction, and undue stress on the caudal cord that interferes with and prevents the cord’s normal ascent, resulting in a low-lying conus medullaris and TCS (Afifi & Bergman, 1998; Pinto et al., 2002). Of all spinal disorders seen by pediatric neurosurgeons, TCS is the most common. A study (Lad et al., 2007) examining data from 9733 patients with TCS who had undergone surgery between 1993 and 2002 showed 71% were 17 or younger. Data indicated TCS had occurred in adult patients also: 18.6% in 18 to 44 year olds, 8.6% in 45 to 64 year olds, and 1.8% in adults older than 65. The clinical presentation of patients with TCS includes a variety of signs and symptoms. The most common presentation frequently includes cutaneous features associated with OSD such as lumbosacral hypertrichosis, cutaneous capillary hemangiomas, midline subcutaneous lipomas, and lumbosacral skin appendages. Other signs and symptoms include neurogenic bladder with the development of incontinence or urinary tract infection, leg or foot weakness, numbness and/or spasticity, unequal leg or foot lengths, deformities of the feet and spine, and nondermatomal back and leg pain (Bui, Tubbs, & Oakes, 2007). In the case of TCS occurring in adults, a subclinical degree of spinal cord traction is present. The traction can become clinically apparent when abrupt cord traction occurs because of sudden flexion of the vertebral canal (e.g., the abdominal flexion movements and trauma resulting from motor vehicle accidents) (Pinto et al., 2002). The sudden traction leads to neurologic deficits caused by anatomic and metabolic changes in the spinal cord. The most common neurologic deficit is pain in the lower back and pain radiating into the lower limbs. This pain is exacerbated by physical activity involving flexion and extension of the trunk. Other findings include sensory deficits in the lower extremities, lower extremity deformities, musculoskeletal deformities such as scoliosis, and urinary incontinence. Adults who present with these types of deficits, but are asymptomatic as children, often are misdiagnosed with “failed back syndrome” or other unrelated problems of the spine (Yamada & Lonser, 2000). The radiologic criteria that have been established to aid in diagnosing this condition are a filum terminale that has a diameter greater than 2 mm or a spinal cord that terminates lower than the L2 or L3 vertebral body levels, and a conus medullaris displaced posteriorly with the filum in contact with the dural sac at or near the L5 lamina (Yamada & Lonser, 2000; Pinto et al., 2002). However, because of considerable anatomic variation of the conus medullaris and filum terminale, the patient’s history, examination, and radiologic findings are important in diagnosing TCS. The surgical treatment for TCS is to perform a laminectomy at the lumbosacral junction, incise the dura and open the arachnoid, and then section the filum and untether the cord. Care must be taken to not cut any spinal roots (Bui, Tubbs, & Oakes, 2007). (See Chapter 7 for additional information on tethered cord syndrome in the adult.) The vast majority (89%) of fila fuse on the dorsal midline of the dura, with 11% fusing to the left or right of the midline. The level of fusion varies anywhere from the lower L5 vertebral body to the upper S3 body. The majority (approximately 85%) of fila fuse at or below S1, and approximately 15% fuse above the S1 level. Forty-four percent of the time the fusion is at the same level as the termination of the dural sac (Hansasuta, Tubbs, & Oakes, 1999). The work of Pinto and colleagues (2002) supports these findings. The diameter of the filum terminale decreases in a superior to inferior direction; the mean thickness at the midpoint of the filum is 0.76 ± 0.39 mm and the mean thickness at the initial point of origin is 1.38 ± 0.56 mm. In addition to the variation in the anatomy of the filum and its relationship with the dural sac, anatomic variations of the conus medullaris are still being documented. The accepted view was that the mean level of termination of the conus medullaris was at the level of the L1-2 intervertebral disc (Reimann & Anson, 1944). Many recent MRI (Table 3-1) (Saifuddin, Burnett, & White, 1998; Macdonald et al., 1999; Malas et al., 2000; Arai et al., 2001; Demiryürek et al., 2002) and cadaveric (Gatonga et al., 2010
General Anatomy of the Spinal Cord
Overview of Spinal Cord Organization
External Morphology
Meninges
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine