CHAPTER 7 The Somatosensory System
The somatosensory system provides information to the central nervous system (CNS) about the state of the body and its contact with the world. It does so by using a variety of sensory receptors that transduce mechanical (pressure, stretch, and vibrations) and thermal energies into electrical signals. These electrical signals are called generator, or receptor, potentials and occur in the distal ends of axons of first-order somatosensory neurons, where they trigger action potential trains that reflect information about the characteristics of the stimulus. The cell bodies of these neurons are located in dorsal root (Fig. 7-1, A; see Fig. 4-8) and cranial nerve ganglia.
SUBDIVISIONS OF THE SOMATOSENSORY SYSTEM
The somatosensory system receives three broad categories of information based on the distribution of its receptors. Its exteroceptive division is responsible for providing information about contact of the skin with objects in the external world, and a variety of cutaneous mechanoceptive, nociceptive (pain), and thermal receptors are used for this purpose. Understanding this division will be the main focus of this chapter. The proprioceptive component provides information about body and limb position and movement and relies primarily on receptors found in the joints, muscles, and tendons. Because these receptors initiate pathways that in part are intimately involved in the control of movement, they will be discussed in Chapter 9; however, the ascending central pathways that originate with them and that underlie conscious and unconscious proprioceptive functions will be covered later in this chapter. Finally, the enteroceptive division has receptors for monitoring the internal state of the body and includes mechanoreceptors that detect distention of the gut or fullness of the bladder.
The somatosensory pathways can also be classified by the type of information that they carry. Two broad functional categories are recognized, each of which subsumes several somatosensory submodalities. Fine discriminatory touch sensations include light touch, pressure, vibration, flutter (low-frequency vibration), and stretch or tension. The second major functional group of sensations is that of pain and temperature. Submodalities here include both noxious and innocuous cold and warm sensations and mechanical and chemical pain. Itch is also closely related to pain and appears to be carried by particular fibers associated with the pain system.
Of great importance experimentally, the afferent fibers that convey these somatosensory submodalities to the CNS are different sizes. Recall that the compound action potential recorded from a peripheral nerve (Table 5-1) consists of a series of peaks, thus implying that the diameters of axons in a nerve are grouped rather than being uniformly distributed. Information about tactile sensations is carried primarily by large-diameter myelinated fibers in the Aα and Aβ classes, whereas pain and temperature information travels via small-diameter, lightly myelinated (Aδ) and unmyelinated (C) fibers. It is possible to block or selectively stimulate a class of axons of particular size, thereby allowing study of the different somatosensory submodalities in isolation.
Innervation of the Skin
Low-Threshold Mechanosensory
To study the responsiveness of tactile receptors, a small-diameter rod or wire is used to press on a localized region of skin. With this technique, two basic types of responses may be seen when recording sensory afferent fibers: fast-adapting (FA) and slow-adapting (SA) responses (Fig. 7-2). They are present in similar quantities. FA fibers will show a short burst of action potentials when the rod first pushes down on the skin, but then they will cease firing despite continued application of the rod. They may also burst at the cessation of the stimulus (i.e., when the rod is lifted off). In contrast, SA units will start firing action potentials (or increase their firing rate) at the onset of the stimulus and continue to fire until the stimulus ends (Fig. 7-2).
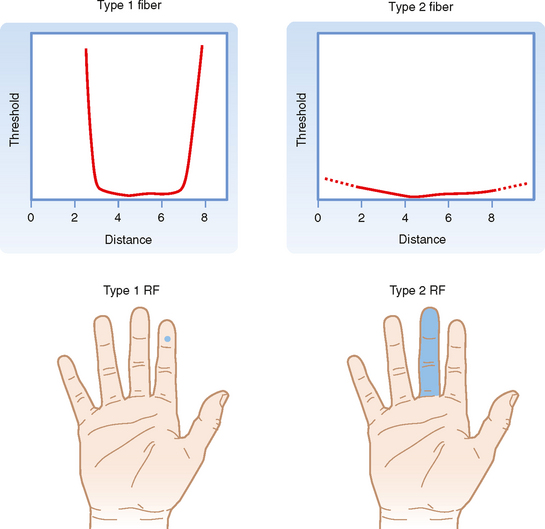
Both the FA and SA afferent classes can be subdivided on the basis of other aspects of their receptive fields, where receptive field is defined as the region of skin from which stimuli can evoke a response (i.e., change the firing of the afferent axon). Type 1 units have small receptive fields with well-defined borders. Particularly for glabrous skin (i.e., hairless skin, such as on the palms of the hands and soles of the feet), the receptive field has a circular or ovoid shape, within which there is relatively uniform and high sensitivity to stimuli that decreases sharply at the border (Fig. 7-3). Type 1 units, particularly SA1 units, respond best to edges. That is, a larger response is elicited from them when the edge of a stimulus cuts through their receptive field than when the entire receptive field is indented by the stimulus.
Type 2 units have wider receptive fields with poorly defined borders and only a single point of maximal sensitivity, from which there is a gradual reduction in sensitivity with distance (Fig. 7-3). For comparison, a type 1 unit’s receptive field typically will cover approximately four papillary ridges in the fingertip, whereas a type 2 unit will have a receptive field that covers most or all of a finger.
Receptive Field Properties
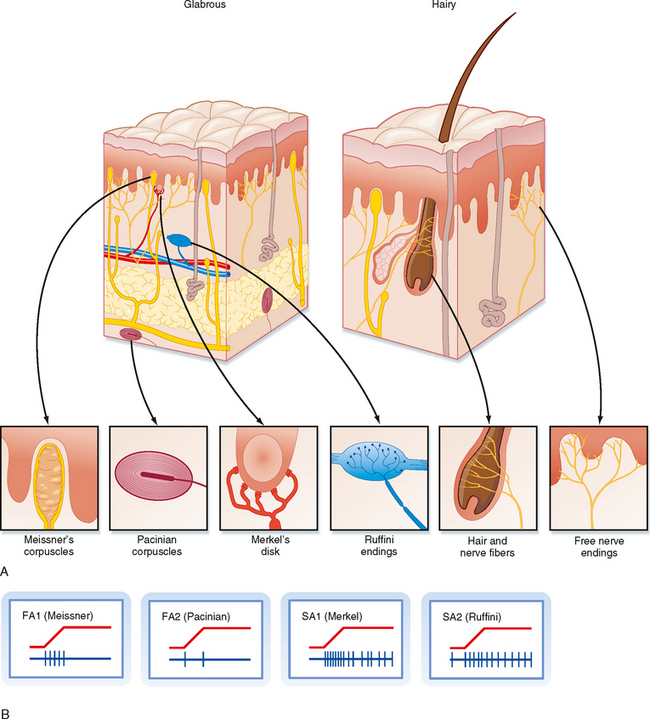
For glabrous skin, the four afferent classes have been associated with four specific types of histologically identified receptor capsules whose locations and physical structure help explain the firing properties of these sensory afferents. FA1 afferents terminate in Meissner’s corpuscles, whereas SA1 afferents terminate in Merkel’s disks. In both cases the capsule is located relatively superficially, either in the basal epidermis (Merkel) or just below the epidermis (Meissner) (Fig. 7-2). These capsules are small and oriented to detect stimuli pressing down on the skin surface just above them, thus allowing SA1 and FA1 afferents to have small receptive fields. For glabrous skin, SA2 afferents terminate in Ruffini’s endings and FA2 afferents end in Pacinian corpuscles. Both these receptors lie deeper in the dermis and connective tissue and therefore are sensitive to stimuli applied over much larger territory. Both Pacinian and Meissner’s capsules act to filter out slowly changing or steady stimuli, thus making these afferents selectively sensitive to changing stimuli.
For hairy skin, the relationship between receptors and afferent classes is similar to that of glabrous skin. SA1 and SA2 fibers connect to Merkel’s and Ruffini’s endings, the same as for glabrous skin. Pacinian corpuscles also underlie the properties of FA2 afferents; however, they are not found in hairy skin but, instead, are located in deep tissues surrounding muscles and blood vessels. There is not an exact analogue to the FA1 afferents. Rather, there are hair units, which are afferents whose free endings wrap around hair follicles (Fig. 7-2). Each such hair unit will connect with about 20 hairs to produce a large ovoid or irregularly shaped receptive field. These units are extremely sensitive to movement of even a single hair. There are also field units that respond to touch of the skin, but unlike FA1 units, they have large receptive fields.
The relationship of the firing rates in the various afferent classes to perceived stimulus quality is another important issue that has been addressed with microneurographic techniques. When a single SA fiber is stimulated with brief current pulses such that each pulse triggers a spike, a sensation of steady pressure is felt at the receptive field area of that fiber. As pulse frequency is intensified, an increase in pressure is perceived. Thus, the firing rate in SA fibers codes for the force of the tactile stimulus. As another example, when an FA fiber is repetitively stimulated, a sensation of tapping results first, and as the frequency of the stimulus is increased, the sensation turns to one of vibration. Interestingly, in neither case does the stimulus change its qualitative character, for example, to a feeling of pain, as long as the stimulus activates only a particular fiber class. This is evidence that pain is a distinct submodality that uses a set of fibers distinct from those used by low-threshold mechanoreceptors.
Innervation of the Body
Axons of the peripheral nervous system (PNS) enter or leave the CNS through the spinal roots (or through cranial nerves). The dorsal root on one side of a given spinal segment is composed entirely of the central processes of dorsal root ganglion cells. The ventral root consists chiefly of motor axons, including α motor axons, γ motor axons (see Chapter 9), and at certain segmental levels, autonomic preganglionic axons (see Chapter 11).
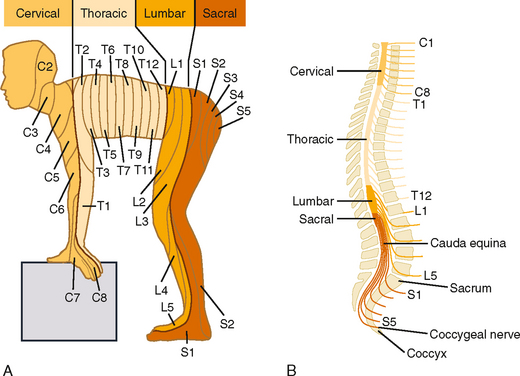
The pattern of innervation is determined during embryological development. In adults, a given dorsal root ganglion supplies a specific cutaneous region, which is called a dermatome. Many dermatomes become distorted during development, chiefly because of rotation of the upper and lower extremities as they are formed, but also because humans maintain an upright posture. However, the sequence of dermatomes can readily be understood if depicted on the body of a person in a quadrupedal position (Fig. 7-4).
Within the dorsal roots, fibers are not randomly distributed. Rather, the large myelinated primary afferent fibers assume a medial position in the dorsal root, whereas the fine myelinated and unmyelinated fibers are more lateral. The large, medially placed afferent fibers enter the dorsal column, where they bifurcate to form rostrally and caudally directed branches. These branches give off collaterals that terminate in the several neighboring segments. The rostral branch also ascends to the medulla as part of the dorsal column—medial lemniscus pathway. The axonal branches that terminate locally in the spinal cord gray matter transmit sensory information to neurons in the dorsal horn and also provide the afferent limb of reflex pathways (see Chapter 9).
The trigeminal nuclear complex consists of four main divisions, three of which are sensory. The three sensory divisions (from rostral to caudal) are the mesencephalic, chief (or main) sensory, and spinal (or descending) trigeminal nuclei. The latter two are typical sensory nuclei in that the cell bodies contained in them are second-order neurons. The mesencephalic nucleus actually contains first-order neurons and thus is analogous to a dorsal root ganglion. The last division of the trigeminal complex is the motor nucleus of the trigeminal nerve, whose motor neurons project to skeletal muscles of the head via the trigeminal nerve (see Fig. 4-7, C-G).
Innervation of the Face
Proprioceptive information is also conveyed via the trigeminal nerve; however, in this unique case, the cell bodies of the first-order fibers are located within the CNS in the mesencephalic portion of the trigeminal nucleus. The central processes of these neurons terminate in the motor trigeminal nucleus (to subserve segmental reflexes equivalent to the segmental spinal cord reflexes—see Chapter 9), the reticular formation, and the chief sensory trigeminal nucleus.
Central Somatosensory Pathways for Discriminatory Touch and Proprioception
Dorsal Column—Medial Lemniscus Pathway
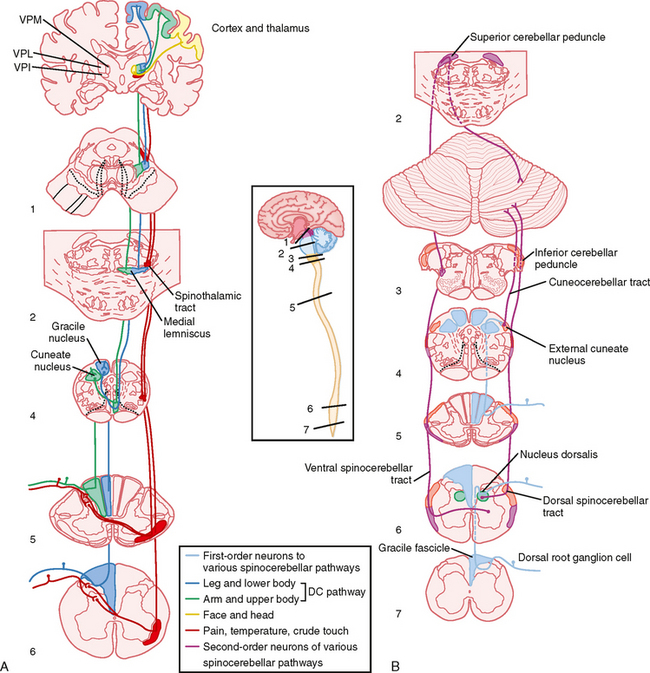
This pathway is shown in its entirety in Figure 7-1, A. The dorsal columns are formed by ascending branches of the large myelinated axons of dorsal root ganglion cells (the first-order neurons). These axons enter at each spinal segmental level and travel rostrally up to the caudal medulla to synapse in one of the dorsal column nuclei: the nucleus gracilis, which receives information from the lower part of the body and leg, and the nucleus cuneatus, which receives information from the upper part of the body and arm. Note that in the dorsal columns and across the dorsal column nuclei there is a somatotopic representation of the body, with the legs represented most medially, followed by the trunk and then the upper limb. This somatotopy is a consequence of newly entering afferents being added to the lateral border of the dorsal funiculus as the spinal cord is ascended. Such somatotopic maps are present at all levels in the somatosensory system, at least through the primary sensory cortices.
The axons of dorsal column nuclear projection neurons exit the nuclei and are referred to as the internal arcuate fibers as they sweep ventrally and then medially to cross the midline at the same medullary level as the nuclei (Fig. 4-7, F). Immediately after crossing the midline, these fibers form the medial lemniscus, which projects rostrally to the thalamus. Knowledge of this decussation level is clinically important because damage to the dorsal column—medial lemniscal pathway below this level, which includes all of the spinal cord, will produce loss of fine somatosensory discriminatory abilities on the same, or ipsilateral, side of the lesion, whereas lesions above this level will produce contralateral deficits. Moreover, because there is a clear somatotopic arrangement of fibers in the medial lemniscus, localized lesions cause selective loss of fine-touch sensations limited to specific body regions.
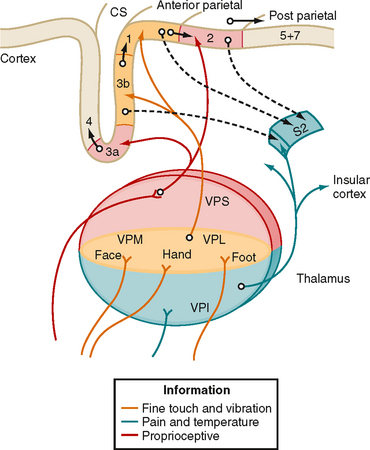
The third-order neurons of the pathway are located in the ventral posterior lateral (VPL) nucleus of the thalamus and project to somatosensory areas of the cerebral cortex (Fig. 7-5).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree