Pulmonary Agenesis
Complete absence of both lungs is extremely unusual and incompatible with life. However, unilateral agenesis, involving one or more lobes, has been seen in 1 in 10,000 to 20,000 autopsies and, in the absence of other severe anomalies, is compatible with long-term survival (
58). There is a 1.3:1 female predominance with unilateral agenesis; the right and left lungs are absent with equal frequency. Associated anomalies are noted in about 75% of cases and include, in decreasing order of frequency, cardiovascular, gastrointestinal, skeletal, and urogenital systems (
59). Cardiovascular malformations include dextrocardia, septal defects, patent ductus arteriosus, and total anomalous pulmonary venous return. Skeletal anomalies include hemivertebrae and a high frequency of thumb malformations, especially triphalangeal thumb (Mardini-Nyhan association) (
60). Along with the radial and vertebral anomalies, imperforate anus and TEF have been described, suggesting an association of pulmonary agenesis with the VATER or VACTERL association (
61). There are also some phenotypic similarities to the syndrome associated with inactivation of ERK/MAP kinase genes MEK1 and MEK2 (
62).
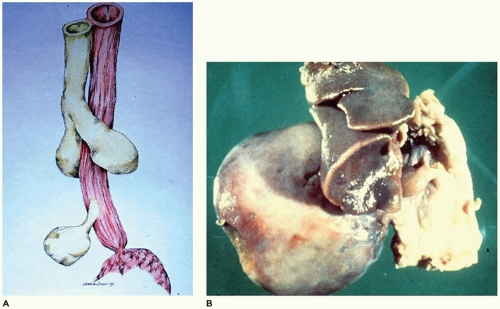
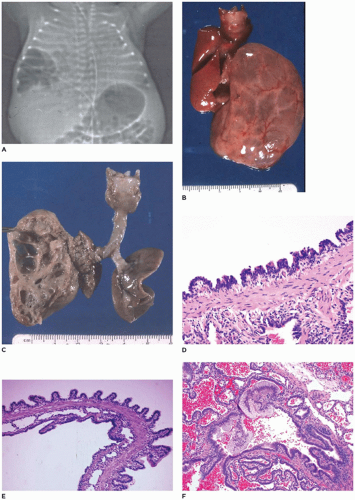
The larynx and upper trachea are usually well-formed in unilateral pulmonary agenesis, although with bilateral agenesis the total trachea may be absent. The lower trachea in unilateral agenesis may continue directly into the existing lung as a tracheobronchus or bifurcate at the carina, giving rise to a rudimentary blind-ending bronchus on the side of the agenesis. The pulmonary artery and vein to the side of the agenesis are absent or hypoplastic and may have an unusual course to the lung, often forming a pulmonary sling (
63). Shift of the mediastinum to the side of the agenesis is usually present, often giving the appearance of dextrocardia in right-sided agenesis. Studies in older infants have demonstrated an absolute increase in the number of alveoli in the existing lung despite a reduced number of bronchial generations and pulmonary artery branches.
Abnormal Lobation, Location, and Shape
Abnormalities of lobation of the lung are usually of little clinical significance unless they are associated with other anomalies such as the asplenia or polysplenia syndrome (discussed earlier in chapter). Lobes may be fused to give the appearance of a single lobe on the right or left, or pleural fissures may produce the appearance of multiple lobes. The appearance of multiple lobes may be seen in infants with long-standing healed bronchopulmonary dysplasia (
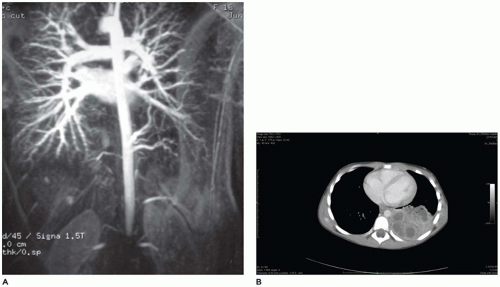
64). Fusion of the lungs in the midline behind the heart produces a conjoined, or “horseshoe,” lung analogous to the horseshoe kidney. Additional anomalies are often present in association with horseshoe lung, including those of the VATER association and the PAGOD syndrome (pulmonary hypoplasia, agonadism, omphalocele, dextrocardia,
and diaphragmatic defect), among others (
65). Bronchial supply to both lungs may be anatomically normal, but there is usually an anomalous pulmonary artery supply and venous drainage resembling that seen in scimitar syndrome.
Herniation of the lung across the mediastinum into the opposite hemithorax, associated with ILO, CPAM, extralobar sequestration, and other conditions, occurs relatively frequently (
1). The lung can also herniate outside the thoracic cavity, usually into the neck. Cervical herniation or “protrusion” is most frequently reported as a “normal variant” in some infants and children. It may also be seen, however, as a result of trauma or surgery, and in association with iniencephalus, the Klippel-Feil syndrome, and the cri du chat syndrome. A familial occurrence has been noted, and the condition is thought to be an autosomal dominant hereditary disease. Herniation through the diaphragm and intercostal spaces may also occur.
Pulmonary Hypoplasia
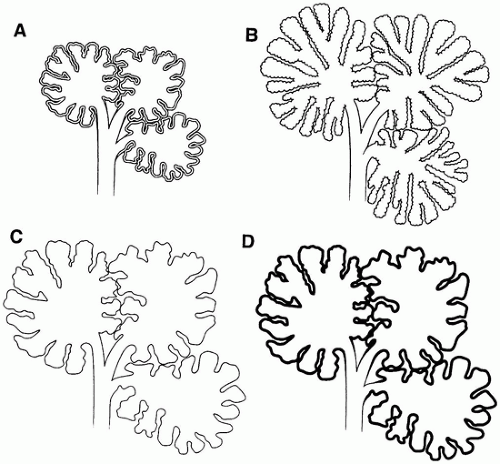
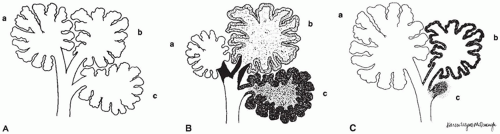
Pulmonary hypoplasia is the incomplete or defective development of the lung resulting in overall reduced size due to reduced numbers or size of acini (
Figure 12-20A). Lung weight and lung weight-to-body weight ratio are the simplest means of determining whether hypoplasia exists. The normal lung weight-to-body weight ratio for term and near-term infants is 0.022 (range 0.012 to 0.025) (
77). Emery and Mithal (
78) describe a radial alveolar count using a line intersect method in which a line is drawn from a terminal bronchiole perpendicular to the nearest septal division or pleura surface (
Figure 12-20B). The number of alveoli intersected by the line determines the count with the mean for term infants of 4.4 ± 0.9 (
79). Alveolar counting and lung volume measurements may also be used to define hypoplasia (
80). Two-dimensional or three-dimensional ultrasound and MRI have also been used in determining whether the lungs of an
in utero fetus or newborn infant may be hypoplastic. However, secondary changes such as bronchopneumonia or pulmonary hemorrhage which increase the weight of the lungs create difficulties in the assessment for the presence of hypoplasia on basis of lung weight alone. One simple but less precise observation is the distance of a terminal bronchiole from the pleural surface.
Pulmonary hypoplasia is noted in more than 10% of neonatal autopsies and occurs in association with another malformation (or malformations) in more than 85% of cases (
81). The most frequently occurring anomalies are diaphragmatic defects and renal malformations (
Table 12-3), but a wide variety of anomalies have been described (
1). The common feature of most of these anomalies is that they directly or indirectly compromise the thoracic space available for lung growth. The cause of the decreased thoracic space may be intrathoracic (e.g., abdominal contents herniated through a defect in the diaphragm) or extrathoracic (e.g., enlarged cystic kidneys). The thorax itself may be abnormal as in Jeune asphyxiating thoracic dystrophy, spondyloepiphyseal dysplasia congenita, and achondroplasia (
82).
In utero accumulation of fluid within the thorax as pleural effusion or chylothorax has also been implicated in the production of pulmonary hypoplasia.
Pulmonary hypoplasia may also occur in the absence of other anomalies or in cases of prolonged preterm premature rupture of amniotic membranes leading to oligohydramnios, loss of fetal cushioning, and lack of amniotic fluid pressure and growth factors which would normally be inhaled into
the lung (
81). As with infants with hypoplasia secondary to other anomalies, these infants present with respiratory distress, are difficult to ventilate, and frequently have episodes of pneumothorax (PT) and interstitial pulmonary emphysema (IPE). Potter sequence with sloping forehead, flattened face and nose, receding chin, large ears, broad spade-like hands, and deformations of the limbs secondary to compression by the uterus in the absence of adequate amniotic fluid is a consistent finding in cases associated with oligohydramnios from any cause. Pulmonary hypoplasia has been noted in children with Down syndrome, but it is thought to result from failure of the lung to develop properly in the postnatal period (
83).
At autopsy, the lungs may be either uniformly reduced in size or markedly asymmetric (e.g., with diaphragmatic hernia). In cases in which the pulmonary hypoplasia is the direct cause of death, the lung weight usually is less than 40% of expected and is often as low as 20% to 30%. Histologically, the acini are small for the infant’s gestational age, but alveolar and capillary development is usually consistent with the gestational age.
Infantile (Congenital) Lobar Emphysema (Overinflation)
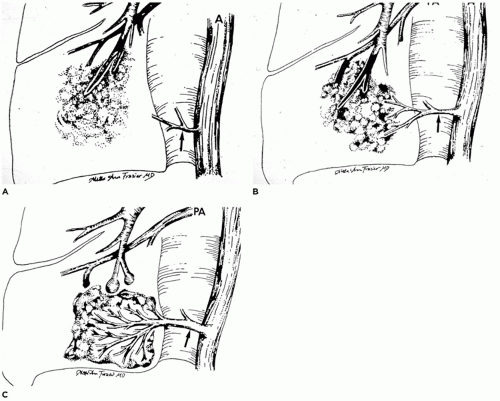
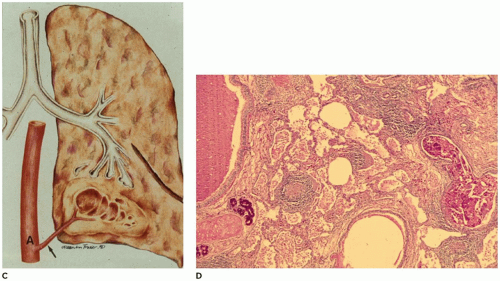
ILO is the overinflation or hyperplasia of a pulmonary lobe as the result of a partial or complete obstruction of the bronchus to the lobe by intrinsic or extrinsic factors (
66) (
Table 12-4). More recently, it is being recognized that not all cases involve the whole lobe; rather a segment is involved, especially on the left side where the upper segments are involved, sparing the lingula. This is especially important to recognize, since these patients may be cured by segmentectomy (
84). Boys are more frequently affected than girls (1.5:1). ILO presents in the first week of life in about 50% of cases (with about 40% presenting in the first day of life) and in the first 6 months of life in over 80%, but ILO can occasionally be seen in children and young adults from 7 months to 20 years of age. Symptoms are those of mild respiratory distress increasing over a period of hours to days to weeks; cyanosis, respiratory infections, vomiting, choking, and feeding difficulties may also be seen. Rarely, the lesion may present as a sudden PT (
66). Imaging studies reveal, in the classic form (see below), a characteristic hyperlucent, overdistended lobe producing mediastinal shift and compression of the uninvolved lobes (
85) (
Figure 12-21A). In the polyalveolar lobe form (see below), imaging may display a lobe of normal lucency but one that occupies a disproportionate part of the hemithorax with mediastinal shift. Less frequently, retained lung fluid may be seen in the involved hyperexpanded lobe (usually the polyalveolar lobe type) on initial examination but which may clear over subsequent days. Associated anomalies are present in 5% to 40% of patients, and 70% of these anomalies are cardiovascular (
86). The upper lobes are involved in over 95% of cases—the left slightly more often than the right. Multiple lobe involvement occurs in about 15% of cases, usually with at least one lobe being an upper lobe. Bilateral involvement has been reported in one case involving the left upper and right middle lobes. Lower lobe involvement is rarely seen except in the “acquired” form of ILO, as in premature infants receiving mechanical ventilation who develop granulation tissue obstruction of a lower lobe bronchus, probably as a result of endotracheal tube suctioning (
87).
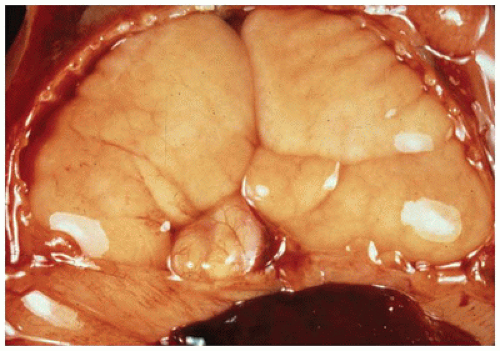
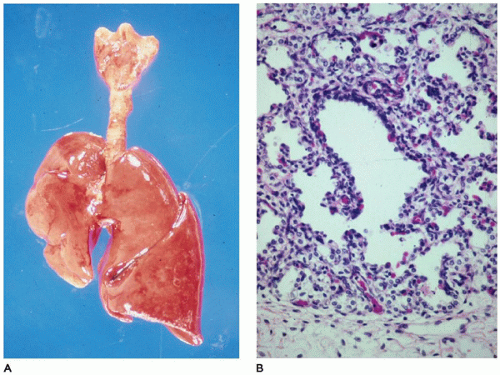
Grossly, the lobe
in vivo and after resection is hyperexpanded with individual alveoli, which may be readily visualized (
Figure 12-21B). Microscopically, two patterns (classic and polyalveolar) are identified. Nearly, 70% (the classic pattern) display a uniform overdistension of apparently normally developed acini with alveolar saccules and alveoli three to ten times the normal size but with radial alveolar counts (RAC) similar to those of age-matched controls (
Figure 12-21C) (
66). There may be focal disruption of alveolar walls. The other 30% (the polyalveolar pattern) show only little overdistension of what appear to be “complex” acini of the type seen in polyalveolar lobes and hyperplastic lungs (
Figure 12-21D), and these have RACs that are two standard deviations beyond the mean of age-matched controls. Seventy-five percent of these infants with polyalveolar lobe present clinically within the first day or two of life and are likely to show radiologic features of retained lung fluid (
88,
89). Examination of the bronchus to the lobe may reveal stenosis, atresia, or intrinsic obstruction, or the bronchus may be unremarkable if extrinsic compression was present. Cartilage abnormalities of the bronchial wall have been described, but special techniques must be employed to demonstrate these changes convincingly.
Surgical resection of the involved lobe is curative, although nonsurgical management has been successful in unusual cases (
85).
Congenital Pulmonary Lymphangiectasis
CPL is a rare, usually fatal disorder that presents in the first hours to days of life (
90); 5% to 10% of affected infants are stillborn. It is characterized by the presence of dilated thin-walled to thick-walled lymphatics within the interlobular septa and beneath the pleura of the lung. CPL may be seen as a primary disorder or as secondary to obstructive cardiovascular lesions, particularly total anomalous pulmonary venous return, but it may occur as part of a generalized lymphangiectasis or as an isolated pulmonary lesion (
91). There is a distinct male predominance of over 2.5:1. Symptoms include cyanosis and acute respiratory distress. Fluid abnormalities including chylothorax, pleural effusion, fetal hydrops, and maternal polyhydramnios have been described
in utero and postpartum (
92). In addition to the 60% of cases with cardiovascular anomalies, CPL is associated with renal malformations, generalized lymphangiectasis, and other anomalies, in another 20% of cases (
1). The diagnosis of CPL in the absence of cardiovascular or other anomalies, should be strongly suspect.
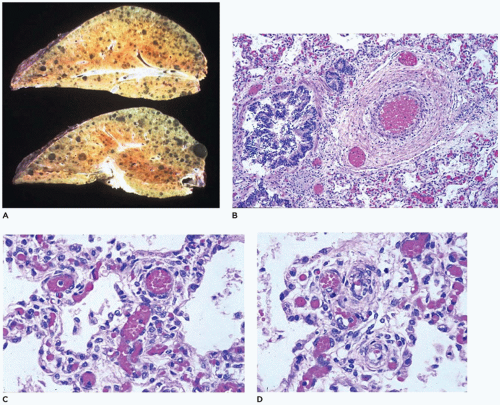
The lungs in CPL are bulky, firm, noncompressible, and covered by a milky network of dilated subpleural lymphatics (
Figure 12-22A). Rarely, a single lobe is involved by this process (
93). On cut section, the lymphatics are fluid-filled and extend from the interconnecting subpleural network into the interlobular septa and around the bronchovascular bundles (
Figure 12-22B). Microscopically, the lymphatics are diffusely
and uniformly dilated and may appear to be increased in number. Identification of lymphatics can be aided by CD31 and D2-40 immunohistochemistry and can help differentiate the lesion from IPE. These small, irregular cysts are lined with a thin layer of endothelial cells and surrounded by a loose myxoid to occasionally dense connective tissue that often contains foci of extramedullary hematopoiesis. Clusters of lymphatics surround bronchovascular bundles within the interlobular septa and may separate acini beneath the pleura (
Figure 12-22C, D). This is in contrast to the air-filled, larger, “unlined” cysts of IPE that are limited to the interlobular septa and do not extend laterally beneath the pleura.
Congenital Pulmonary Airway Malformation
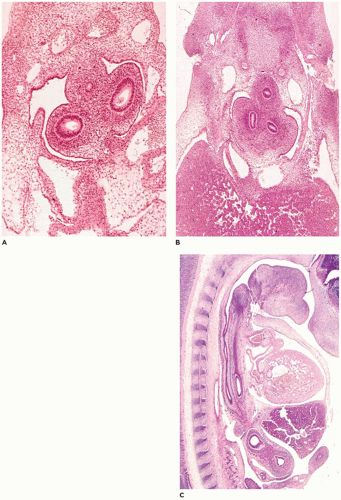
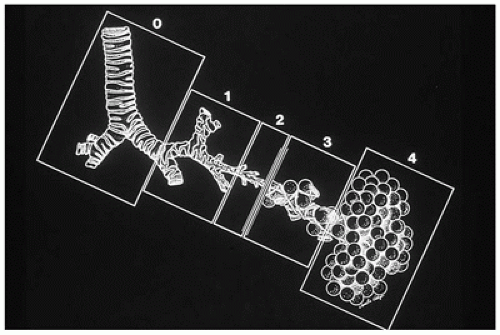
CPAM is a developmental anomaly of the lung, with an incidence of about 1 in 5000 live births, that can be separated into four major types based on clinical and pathologic features (
Figure 12-23) (
94). The former designation “CCAM” was changed to “CPAM” to reflect the fact that the lesions as described below are “cystic” in only two of the four types and “adenomatoid” in only one type (type 3). CPAM more accurately encompasses three types in this classification. CPAMs are the most common surgically resected pulmonary malformation in children. CPAM is a unilateral lesion in about 95% of cases and involves a single lobe in 80% to 90% of cases. The right and left sides of the lung are nearly equally involved, with the lower lobes affected in about 60% of cases.
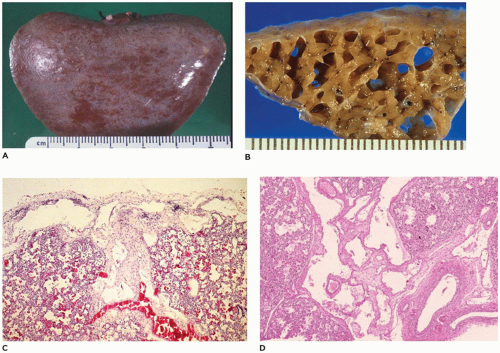
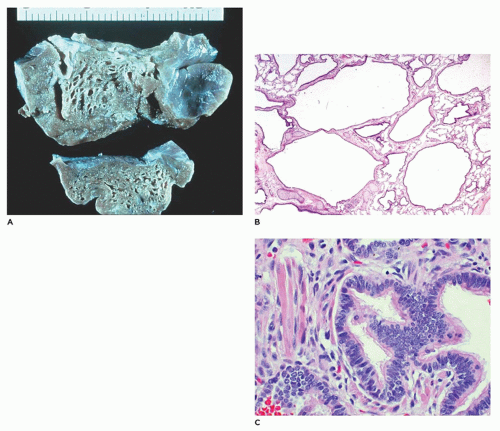
CPAM, type 0, also known as acinar dysplasia or agenesis, is a rarely occurring, infrequently described malformation that is largely incompatible with life (
95). It may be regarded as the most extreme form of pulmonary hypoplasia. It is seen in term and premature infants who are cyanotic at birth and survive only a few hours and is associated with cardiovascular abnormalities and dermal hypoplasia. Grossly, the lungs are small and firm and have a diffusely granular
surface (
Figure 12-24A). Microscopically, tissue consists of bronchus-like structures with muscle, glands, and numerous cartilage plates (
Figure 12-24B). Prominent mesenchymal tissue separates these structures and contains extramedullary hematopoiesis, large thin-walled vascular channels, and collections of amorphic basophilic debris. Rarely, structures resembling proximal bronchioles are present, along with a few scattered acini at the periphery of the lesion.
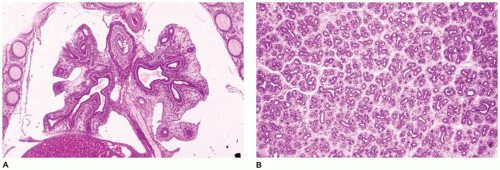
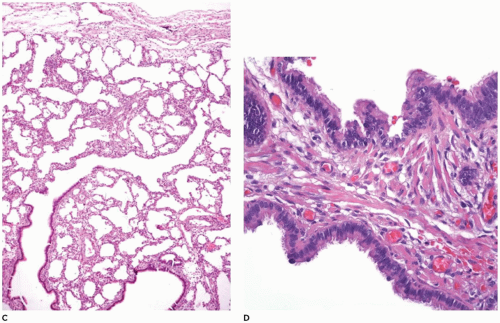
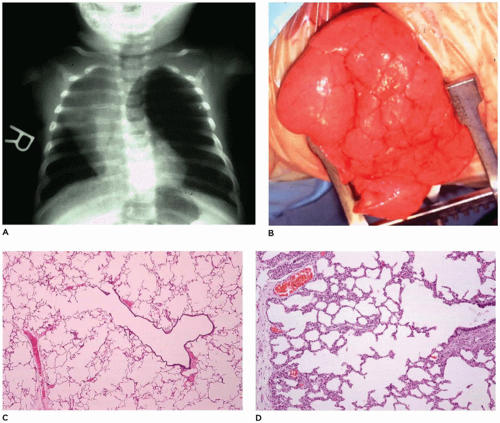
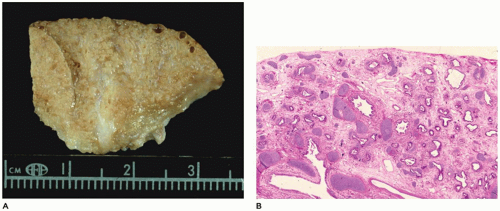
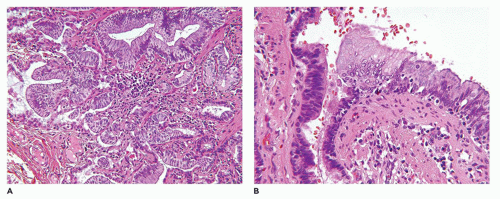
CPAM, type 1, the large or predominant cyst type, presents primarily within the first week to month of life but can be seen in older children and even young adults (
Figure 12-25A). It accounts for nearly 65% of cases and is usually readily amenable to surgery with a good prognosis. Grossly, the type 1 lesion is characterized by single or multiple large cysts (3 to 10 cm in diameter) surrounded by smaller cysts and compressed normal parenchyma (
Figure 12-25B, C). Microscopically, the larger cysts are lined with ciliated, pseudostratified columnar epithelium and the smaller ones by cuboidal to columnar epithelium (
Figure 12-25D, E). More than 45% of the cases display segments of mucus-producing cells among the epithelial lining of the larger cysts or in bronchioles and alveolar duct-like structures adjacent to the larger cysts (
Figure 12-25F). These mucous cells are recognized to be precursors for adenocarcinomas (
Figure 12-26) (see below—Tumors) that have been described in association with type 1 CPAM (
96,
97). The walls of the CPAM, type 1 cysts are composed of elastic tissue overlying fibromuscular connective tissue, and in 5% to 10% of cases, cartilage plates.
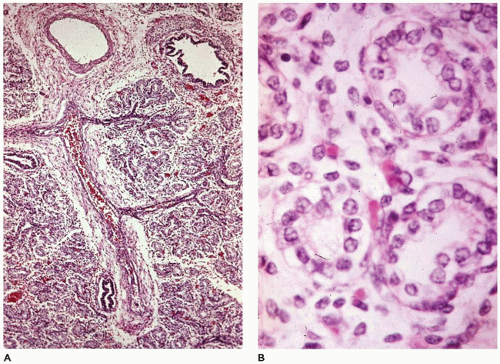
CPAM, type 2, the medium cyst type, accounts for 10% to 15% of cases, is seen exclusively within the first year of life and has a poorer outcome owing to its more frequent association with other anomalies, some of which are incompatible with life (e.g., renal agenesis). The type 2 lesion is composed of cysts 0.5 to 2.0 cm in diameter (rarely larger) that are evenly distributed and blend with the adjacent normal parenchyma (
Figure 12-27A). The cysts occasionally surround normal appearing bronchi. The typical back-to-back bronchiole-like structures are lined by cuboidal to columnar epithelial cells with a thin underlying fibromuscular layer (
Figure 12-27B).
Mucous cells and cartilage plates are absent except as components of “entrapped” normal bronchi. A variant or subgroup of the type 2 lesion, termed rhabdomyomatous dysplasia, contains ribbons of striated muscle fibers throughout the lesion, both in association with the cysts and between alveolar ducts and around blood vessels (
Figure 12-27C). The cysts of this rhabdomyomatous variant may be less prominent than other type 2 lesions. Rhabdomyosarcoma has been reported to originate from CPAM, but this likely represents a primary pleuropulmonary blastoma (PPB) rather than being secondary to CPAM. CPAM, type 2-like features are present in 50% of extralobar sequestrations (
67).
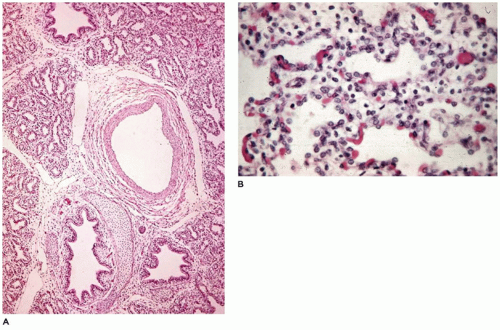
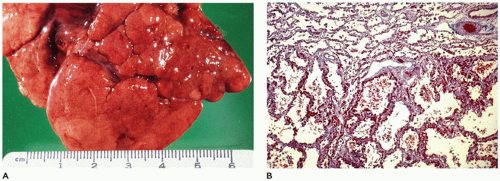
CPAM, type 3, the small cystic or solid type, occurs infrequently (5% of cases), is seen exclusively in the first days to month of life, has a notable male predominance, and owing to its large size and association with maternal polyhydramnios and fetal anasarca, has a high mortality rate. Increased maternal levels of serum alpha-fetoprotein have been anecdotally noted in the second trimester in association with CPAM type 3. CPAM, type 3, the original lesion described by Ch’in and Tang (
98), consists of a large, bulky, parenchymal mass involving an entire lobe or even an entire lung (
Figure 12-28A, B). The mass effect of the lesion consistently produces mediastinal shift and often results in hypoplasia of the uninvolved lung. Cysts are rarely larger than 0.2 cm in diameter, with the exception of scattered larger bronchiole-like structures. Microscopically, the lesion resembles an immature lung devoid of bronchi. Irregular, stellate-shaped, bronchiole-like structures lined with cuboidal epithelial cells are surrounded by alveolar ductules and saccules that are also lined by cuboidal cells, imparting the “adenomatoid” appearance for which this lesion was originally named (
Figure 12-28C). Mucous cells, cartilage, and rhabdomyomatous cells are not present, and there is a paucity of vessels within the lesion.
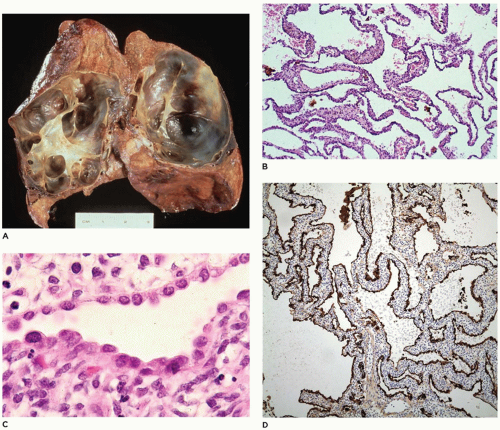
CPAM, type 4, the peripheral acinar cyst type, is composed of large cysts lined by pneumocytes and it is very important to recognize it as the purely cystic pleuropulmonary blastoma (type 1 PPB). It is recommended that all children should be tested for DICER 1 mutation which is the first hit (of two-hits, see below) and is present in 70% of PPBs including those that are nonrecurring. Complete resection and close long-term follow-up are necessary (
97). Once type 1 PPB has been excluded, CPAM type 4 is indeed rare if it exists at all. It is seen equally in boys and girls, with an age range of newborn to 4 years and accounts for 10% to 15% of cases. Before the recognition of type 1 PPB, most of these cases were included in the CPAM type 1 category. Clinically, the type 4 lesions may present with mild respiratory distress, sudden respiratory distress from tension PT, pneumonia, or on occasion, as an incidental finding with no symptoms (
94). Radiographically, the lesion displays large air-filled cysts with mediastinal shift. The lesion involves a single lobe in about 80% of cases and rarely may be bilateral. Grossly, large thin-walled cysts are present at the “periphery” of the lobe and appear to be lined by a smooth membrane (
Figure 12-29A). Microscopically, the cysts are lined by flattened
epithelial cells (type I and II pneumocytes), with occasional low cuboidal epithelium seen (
Figure 12-29B to D). The wall of the cyst is composed of loose mesenchymal tissue with prominent arteries and arterioles. It must be emphasized that PPB type 1 and CPAM, type 4 are one in the same and should be viewed and managed as PPB.
Ultrasonography has been demonstrated to be a highly useful modality in the
in utero diagnosis of CPAM.
In utero serial sonography has demonstrated the gradual reduction in the size of CPAM, type 1 and 2, with subsequent normal development of the uninvolved lung (
99,
100). Anomalies are noted in 15% to 20% of all cases of CPAM, particularly in association with the type 2 lesion (
94,
101) including bilateral renal agenesis/dysgenesis, extralobar pulmonary sequestration, cardiovascular malformation, diaphragmatic hernia, hydrocephalus and macrocephaly, myelomeningocele, jejunal atresia, prune belly syndrome, sirenomelia, bilateral nephromegaly, Pierre Robin syndrome, pulmonary hypoplasia, skeletal malformation, bile duct hypoplasia, left heart hypoplasia, polycytosis of a solitary medial kidney, and congenital nephrotic syndrome (diffuse mesangial sclerosis).
Congenital Alveolar Capillary Dysplasia
Congenital alveolar capillary dysplasia with or without misalignment of pulmonary veins (ACD/MPV) is a uniformly fatal rare entity that presents as respiratory distress, progressive hypoxemia, and pulmonary hypertension in the newborn (
102). Familial occurrence has been noted (
103); most cases are sporadic, with familial cases showing paternal imprinting (
Table 12-5). Many cases have been reported to harbor microdeletions in the
FOX gene cluster on 16q24.1 and mutations in the
FOXF1 gene, a transcription factor that is involved in organogenesis (
104). It is characterized by the failure of formation and ingrowth of alveolar capillaries. Broad alveolar septa with large alveolar capillaries within the septal wall are the hallmark of this disorder (
Figure 12-30A to D) (
105). Capillaries are centrally placed well beneath the basement membrane of the alveolar lining cells and surrounded by loose mesenchyme (
Figure 12-30C). Ectatic veins are present within bronchovascular bundles, occasionally within the adventitia of the pulmonary arteries, and may form an intermittent ring around the bronchiole (
Figure 12-30B). Small muscularized arteries are also present within the acini, extending to the precapillary area (
Figure 12-30D). Other changes include paucity of alveolar capillaries and abnormal capillary size and location, seen as centrally located thin-walled dilated and congested alveolar wall vessels. Special stains including Movat pentachrome, trichrome, elastic, CK7 (for pulmonary epithelium), CD31 (for microvasculature), and SMA (for vascular smooth muscle) may be used to highlight the constellation of histologic changes. High capillary apposition and density may correlate with survival beyond the neonatal period. About a third of the patients with ACD/MPV also have pulmonary lymphangiectasis (
106,
107) and about 80% have anomalies of other organ systems (
108). Treatment with inhaled nitric oxide and ECMO has prolonged life but has been uniformly unsuccessful in changing the fatal outcome of this disorder without lung transplantation.
Acute Lung Injury and Respiratory Distress Syndrome
Acute lung injury is a pattern of diffuse alveolar damage (DAD) seen in association with clinical acute respiratory distress syndrome (RDS). Previously referred to as hyaline membrane disease (HMD), acute lung injury is characterized by firm, atelectatic lungs with an uneven air expansion pattern, focal hemorrhage, edema fluid in alveoli, and hyaline
membranes along terminal and respiratory bronchioles and alveolar ducts. In the pediatric age group, DAD has been typically described in neonates with surfactant deficiency, although other causes include infections, inhalational injury, sepsis, and systemic shock. Breathing difficulty is common in the early neonatal period, occurring in up to 7% of newborn infants, and is not synonymous with RDS. Other common causes of breathing difficulty in term newborn infants include transient tachypnea of the newborn, pneumonia, meconium aspiration syndrome (MAS), persistent pulmonary hypertension of the neonate, and PT (
111).
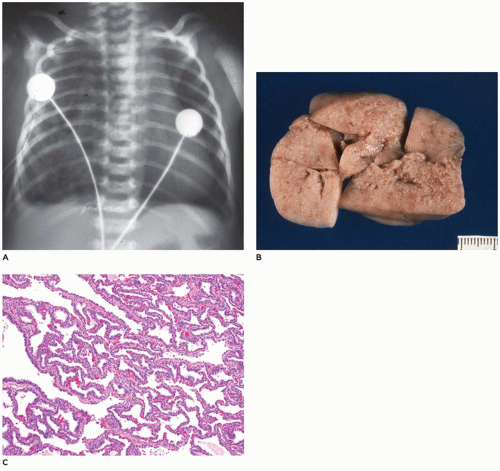
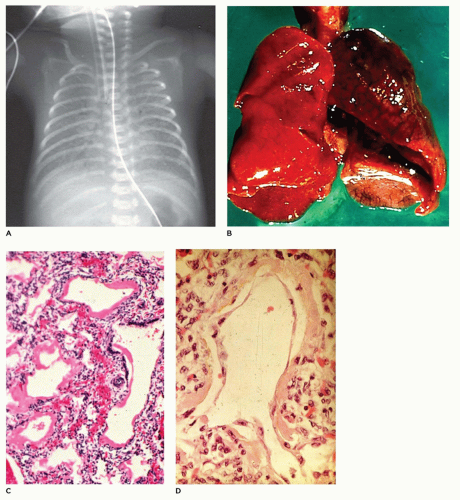
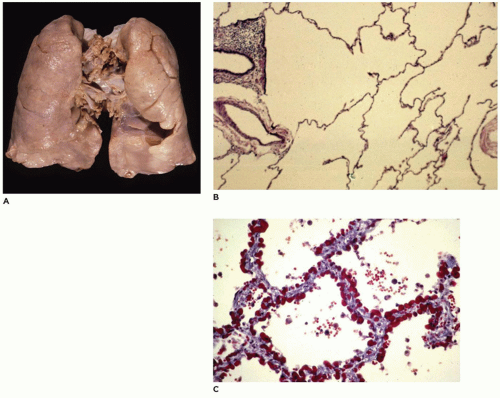
Infants present with tachypnea, intercostal retractions, and hypoxemia and display a typical x-ray image of ground-glass alterations of the lungs with an air bronchogram and diffusely scattered reticulogranular opacities (
Figure 12-32A) (
1). Grossly, the lungs are firm and resemble the liver more than the lung. Microscopically, there is an uneven air expansion pattern with atelectatic acini and
dilated bronchioles and alveolar ducts (
Figure 12-32B). Scattered foci of alveolar hemorrhage and edema are present, but most striking is the presence of smooth, homogeneous, pink membranes lining terminal and respiratory bronchioles and alveolar ducts, particularly at points of division or branching (
Figure 12-32C). These hyaline membranes are composed of necrotic alveolar lining cells, plasma transudate, inhaled amniotic fluid including squames and fibrin. Hemorrhage is often present. Hyaline membranes may be seen in infants who die as early as 3 to 4 hours after birth and are uniformly present as well-formed structures by 12 to 24 hours in infants with RDS. In the absence of severe disease requiring high oxygen tensions and ventilatory pressures, at 36 to 48 hours, the membranes begin to organize and separate from the underlying wall to be replaced by alveolar lining cells or bronchiolar cuboidal or columnar epithelium (
Figure 12-32D). Bacteria may alter the appearance of the membranes by producing fragmented, faintly basophilic structures, with organisms often readily demonstrable by Gram stain on or within the membranes. Conditions associated with hyperbilirubinemia (e.g., kernicterus, intraventricular hemorrhage, intrahepatic bile stasis, disseminated intravascular coagulation) may produce, in infants surviving 3 or more days, yellow hyaline membranes as a result of the presence of unconjugated bilirubin.
Surfactant replacement therapy, although radically decreasing the incidence of acute lung injury in premature infants and its morbidity and mortality in these infants, does not appear to alter the pathologic features of DAD in infants dying of RDS. However, treated children may show a slightly higher incidence of pulmonary hemorrhage and a lower incidence of IPE and PT (
112). Surfactant therapy appears to accelerate the rate of epithelial cell regeneration (
113).
The pathophysiology of acute RDS in the neonate has been divided into eight categories: alveolar fluid transport, surfactant, innate immunity, apoptosis, coagulation, direct alveolar epithelial injury by bacterial products, ventilator-associated lung injury, and repair. The biological baseline network of genes and protein expression is reportedly different in children with RDS, as compared to adults (
114). Beyond the neonatal period, causes of RDS are similar to those in adults. In a prospective, multicenter, observational study covering 21 pediatric intensive care units, pneumonia and sepsis were the most common causes of acute RDS; the authors found a lower acute RDS incidence and mortality in children than those reported for adults (
115). In a masked, randomized, placebo-controlled trial in 24 children’s hospitals in six different countries, surfactant did not improve outcomes relative to placebo in children with direct lung injury/acute RDS (
116).
Bronchopulmonary Dysplasia (Chronic Lung Disease of Prematurity)
Bronchopulmonary dysplasia was first described in 1967 by Northway et al. (
117). In a retrospective study, they described the clinical and pathologic features of 19 infants dying following mechanical ventilation with high concentrations of oxygen for severe HMD (RDS). The pathology was correlated with clinical and radiographic findings and included a 2- to 3-day period of acute RDS, followed by a weeklong period of “regeneration,” another 10-day period of transition to chronic disease, and a final period of chronic disease extending beyond 1 month of life. The pathologic features in the first stage included the typical findings of HMD (e.g., atelectasis, uneven air expansion pattern, hemorrhage, and hyaline membranes). During the second stage, there was necrosis of bronchiolar and alveolar epithelium with persistence of hyaline membranes (
Figure 12-32A to C). In the transition to chronic disease, injury to alveolar epithelium continued, along with widespread bronchial and bronchiolar mucosal metaplasia and marked mucus secretion. Clusters of hyperexpanded alveoli alternated with areas of atelectasis.
In the chronic stage, bronchioles displayed marked peribronchiolar smooth muscle hypertrophy associated with clusters of “emphysematous alveoli.” The birth weights of the 19 infants dying of bronchopulmonary dysplasia varied from 900 to 2466 g, with two-thirds of them weighing more than 1300 g. Bonikos et al. (
118) suggested that bronchopulmonary dysplasia was due to the toxic effects of oxygen, poor bronchial drainage, and the effects of mechanical ventilation. In the 10 years following that initial brief description of the pathology of BPD, a number of other studies described in more detail the pathologic features including the changes noted in alveoli, airways, lymphatics, vessels, and connective tissue as criteria for the staging of bronchopulmonary dysplasia. In 1976, Bonikos et al. (
119) described a severe necrotizing bronchiolitis in the acute stages of BPD and implicated prolonged exposure to high levels of oxygen as a major feature in the cause of bronchiolitis. Since that time, a number of additional factors, including infection, inflammation, poor nutrition, dehydration, and others, have been implicated in the pathogenesis of BPD (
120). In addition to the bronchiolitis, there is a prominent alveolar septal fibrosis in the healed stages along with an increased incidence of cardiac hypertrophy.
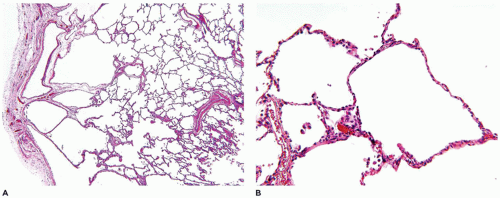
The sequelae of this necrotizing bronchiolitis were described by Stocker in 1986 in a series of 28 patients with long-standing “healed” bronchopulmonary dysplasia, who died at 3 to 40 months of age (
64). Noting the presence of deep pleural fissures and acini with varying degrees of alveolar septal fibrosis (
Figure 12-33), it was suggested that the necrotizing bronchiolitis seen in the acute phases, while prohibiting adequate ventilation, often served to “protect” acini from damage by mechanical ventilation or high levels of oxygen (
Figure 12-34A to C). Stocker also suggested that the alveolar fissures might represent areas of complete loss of acini corresponding to the marked decrease in internal surface area and number of alveoli noted by Sobonya et al. (
121). The 6- to 10-fold reduction in number of alveoli suggested not only an absolute loss of some acini but a generalized reduction in lung growth. In 1991, Margraf et al. (
122) confirmed the reduction in lung volume and small airway density noted by Sobonya.
In recent years, with the advent of surfactant replacement therapy and increased sophistication in the use of mechanical ventilation (including high frequency jet ventilation) and oxygen supplementation, another stage in the evolution of the pathology of bronchopulmonary dysplasia has been seen. Although the occasional case of “classic” acute bronchopulmonary dysplasia with necrotizing bronchiolitis, alveolar cell hyperplasia, and peribronchiolar and alveolar septal fibroplasia is still seen along with focal alveolar septal fibrosis in the older patient (
Figure 12-35A to D), the few infants who now die from bronchopulmonary dysplasia display what might best be described as “acinar simplification.” These simplified acini are characterized by uniformly dilated alveoli whose walls consist of thin alveolar septa with little or no interstitial fibrosis (
123).
The bronchioles are similarly unremarkable, with only an occasional mild increase in peribronchiolar musculature. These changes seem to represent an “arrest” of development of the acini, with a resulting markedly decreased number of alveoli within each acinus (
Figure 12-36A to C). As a result, the surface area of the lung is significantly decreased even in the absence of significant pathology (e.g., alveolar septal fibrosis).
Although high concentrations of oxygen over prolonged periods of time are known to cause alveolar cell hyperplasia and necrotizing bronchiolitis with resulting alveolar septal fibrosis,
it is possible that low levels of oxygen (25% to 35%), while not producing significant alteration in the epithelial lining of the lung or not causing damage sufficient to cause septal fibrosis, may, in very immature infants, inhibit growth of the lung, that is, the development of new alveolar ducts and alveoli. Although the lung appears to “mature” and alveolar septa appear to thin and expand to resemble the septa of term infants, there is no accompanying significant increase in the surface area of the lung through an increase in number of alveoli. Thus, although recent advances in mechanical ventilation have limited the amount of injury to the bronchiole (i.e., no necrotizing bronchiolitis), the continued patency of all bronchioles throughout the course of therapy allows equal injury or inhibition of growth to all acini from even low levels of oxygen therapy.


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access