Chapter Nineteen. The renal tract
CHAPTER CONTENTS
Introduction 259
Kidney functions 259
Renal function 262
The production of urine 262
Electrolytes 262
Diffusion 262
Osmosis 262
Hydrostatic pressure 263
pH and acid–base balance 263
Glomerular filtration 263
Tubular reabsorption and secretion 264
Transport mechanisms in the nephron 264
Regulation of urine concentration and volume 265
The renin–angiotensin–aldosterone system 266
Natriuretic hormone 266
Introduction
The structure and function of the renal tract and how these change in pregnancy will be discussed within this chapter. Although the production of urine is also discussed, fluid and electrolyte balance and the regulation of acid–base balance are discussed in Chapter 20 in an attempt to integrate the roles of the respiratory and renal systems. This should be of value to the reader interested in the interactions between systems and may avoid turning backwards and forwards in the text to synthesise material. The role of renin and the angiotensin–aldosterone system and the control of blood pressure will be discussed in Chapter 20.
Kidney functions
The kidneys play a major role in maintenance of homeostasis within the internal environment by their regulation of the volume and composition of the body fluids. Each day the kidneys filter several litres of fluid from the bloodstream, ensuring that toxins, metabolic wastes and excess ions are excreted from the body in urine. Other than the excretory function, the roles of the kidney are:
• Regulation of the volume and chemical make-up of the blood.
• Maintenance of balance between water and salts, acids and bases.
• Production of the enzyme renin, which helps to regulate blood pressure, and production of the hormone erythropoietin, which stimulates red cell production in the bone marrow.
• Conversion of vitamin D to its active form.
Also part of the renal system are the two ureters, which convey urine to the urinary bladder where urine is stored until it is voided through the urethra.
Anatomy of the kidney
The kidneys are paired, compact organs situated on either side of the vertebral column between the 12th thoracic and the 3rd lumbar vertebrae. They are situated behind the peritoneum and are attached to the posterior abdominal wall by adipose tissue. An adult kidney is bean-shaped with a convex lateral surface and concave medial surface. A cleft in the medial surface is called the hilum and leads to a space within the kidney called the renal sinus. The hilum is the site of entry and exit of structures that include the ureters, renal blood vessels, lymphatics and nerves. Each kidney weighs about 150 g and measures 12 cm long, 6 cm wide and 3 cm thick. The adrenal gland sits on top of the kidney.
Structure
Three layers of supporting tissue surround each kidney:
1. The renal capsule is closest to the kidney and is fibrous and transparent. This is a strong barrier that prevents infections in nearby regions spreading to the kidneys.
2. The adipose capsule is a middle layer of fatty tissue that helps hold the kidney in place and protects it from trauma.
3. The renal fascia is the outermost covering and is made of dense fibrous connective tissue that surrounds both kidney and adrenal gland and anchors them to surrounding structures.
Beneath the capsule lie three distinct regions: the outer cortex, the medulla and the inner renal pelvis (Fig. 19.1). The cortex has a light granular appearance. The medulla is darker and reddish brown with cone-shaped masses of tissue called medullary or renal pyramids. The base of each pyramid is broad and faces the renal cortex while the pointed apex ( papilla) projects into a minor calyx. Several minor calyces open into each of two or three major calyces, which then open into the renal pelvis. The pyramids have a striped appearance because they consist of bundles of microscopic tubules. The renal columns are extensions of cortical tissue that separate the pyramids. Each medullary pyramid and its cap of cortical tissue is known as a lobe of the kidney. There are usually between 8 and 18 lobes in a kidney.
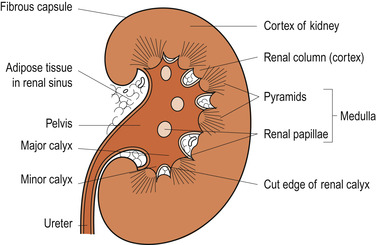 |
| Figure 19.1 Coronal section through a kidney. (From Hinchliff S M, Montague S E 1990, with permission.) |
The renal pelvis
The renal pelvis is a flat funnel-shaped tube that is continuous with the ureter. The urine produced by the kidney flows continuously from the papillae into the calyces and down the ureter where it is then stored in the bladder. The walls of the calyces, pelvis and ureter contain smooth muscle, which contracts in peristaltic movements to propel urine towards the bladder.
Microscopic structure of the kidney
Each kidney contains over 1 million nephrons, which are the functional units of the kidney. Each nephron consists of a renal tubule and a tuft of blood vessel capillaries called the glomerulus. The end of the tubule, called a Bowman’s capsule, is enlarged and invaginated to hold the glomerulus. The outer or parietal layer of the Bowman’s capsule is composed of simple squamous epithelium and has a purely structural function. The inner or visceral layer that clings to the glomerulus is made up of branching epithelial cells called podocytes which form part of the filtration membrane. The branches of the podocytes end in pedicles or foot processes. The clefts between the pedicles form filtration slits or slit pores.
The capillary endothelium of the glomerulus is porous, which allows large quantities of solute-rich fluid to pass from the blood into the glomerular capsule. This fluid is called the filtrate and is processed by the renal tubules to form urine. A basement membrane divides the endothelium of the capillary from the epithelium lining the Bowman’s capsule. The Bowman’s capsule and its contained glomerulus are known as a renal corpuscle and are situated in the renal cortex. The structure comprising the capillary endothelium, basement membrane and podocytic epithelium constitutes the selective filtration barrier.
The remainder of the renal tubule is about 3 cm long and can be divided into four anatomically distinct regions: the proximal convoluted tubule, the loop of Henle, the distal convoluted tubule and the collecting duct (Fig. 19.2).
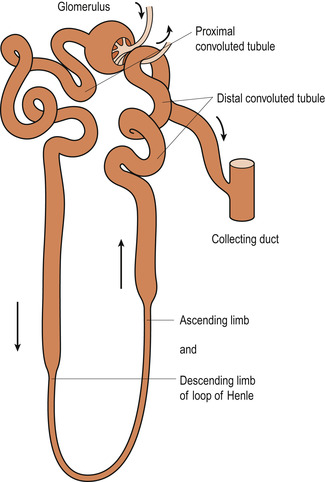 |
| Figure 19.2 Microanatomy of nephron. (From Hinchliff S M, Montague S E 1990, with permission.) |
The proximal convoluted tubule extends about 16 mm through the cortex. This region of the tubule is lined by large columnar epithelial cells, which have a brush border of microvilli on their internal surface for solute reabsorption.
The loop of Henle has a descending limb and an ascending limb. The thin-walled descending limb extends from the proximal convoluted tubule, dips down into the medulla and makes a U turn, moving back into the cortex by the thick-walled ascending limb. In this loop the columnar cells are flatter and contain fewer microvilli on their luminal (side facing into the lumen) surfaces.
The distal convoluted tubule, continuous with the loop of Henle, is comparatively short (about 4–8 mm) and leads into the collecting ducts, which fuse together as they approach the renal pelvis to form papillary ducts. These ducts open at the tips of the medullary papillae to discharge their urine into the calyces and renal pelvis. The first part of the distal tubule folds back to bring it nearer to the afferent arteriole. This forms the juxtaglomerular apparatus (see below).
Cortical and juxtamedullary nephrons
About 85% of the nephrons are called cortical nephrons because they are situated in the cortex (except where their loops of Henle dip into the medulla). The remaining 15% of nephrons are different in structure and are called juxtaglomerular nephrons. They are located near the cortex–medullary junction and their loops of Henle are found deep in the medulla. Their thin segments are more extensive than those of the cortical nephrons. The juxtamedullary nephrons have long thin-walled looping capillaries, called the vasa recta, running parallel with their loops of Henle.
Capillary beds of the nephron
Every nephron is closely associated with two capillary beds which form the microvasculature of the nephron. These are the glomerulus and the peritubular capillary bed. The glomerulus is unlike any other capillary bed because it is fed and drained by arterioles. Glomeruli originate from an afferent arteriole arising from interlobular arteries that permeate the renal cortex and drain into efferent arterioles. The peritubular capillary bed consists of capillaries arising from the efferent arterioles draining the glomeruli. These capillaries cling closely to the renal tubules and empty into nearby venules. Just as the glomerular capillary bed is adapted for filtration, the peritubular bed is adapted for reabsorption. They are low-pressure porous capillaries. The additional vessels of the vasa recta play a part in reabsorption of salts.
The blood pressure within the glomerular capillary bed is very high for two reasons:
1. Arterioles are high-resistance vessels.
2. The afferent arteriole has a much larger diameter than the efferent arteriole.
This high pressure forces fluids and solutes out of the glomerular blood along its entire length into the Bowman’s capsule. About 99% of this filtrate is reabsorbed into the blood in the peritubular capillary beds. As blood flows into the renal circulation, it encounters high resistance, first in the afferent and then in the efferent arterioles. Renal blood pressure declines from 95 mmHg in the renal arteries to 8 mmHg in the renal veins. The resistance of the afferent arterioles protects the kidney from large fluctuations in the systemic blood pressure. Resistance in the efferent arterioles maintains the high glomerular pressure and reduces the hydrostatic pressure in the peritubular arteries to facilitate reabsorption.
The juxtaglomerular apparatus
The juxtaglomerular apparatus is a region found in each nephron where the distal convoluted tubule lies against the afferent arteriole as it supplies the glomerulus (Fig. 19.3). Where the two parts of the nephron touch, the cellular structures are modified. The afferent arteriolar wall contains juxtaglomerular cells. These are enlarged smooth muscle cells that contain granules filled with renin. They seem to be mechanoreceptors responding to the blood pressure in the afferent arterioles. The macula densa is a group of tall, closely packed distal tubule cells that act as chemoreceptors or osmoreceptors responding to sodium chloride concentration in the distal tubule. These two types of cell are important in the regulation of filtrate formation and systemic blood pressure.
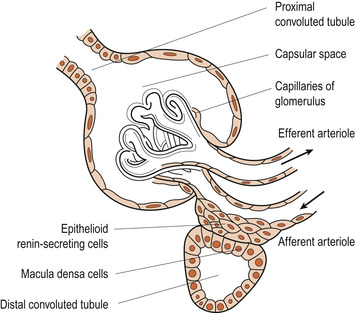 |
| Figure 19.3 The juxtaglomerular apparatus showing the macula densa. (Redrawn from Creager 1983.) |
Blood supply
About 25% of cardiac output is delivered each minute to the kidneys. This is a higher blood supply than to any other tissue. The two renal arteries arise high up on the abdominal aorta and enter the hilum, dividing in the renal tissue to form interlobar arteries between the pyramids. Arcuate arteries arise here and give rise to interlobular arteries, which branch to form the afferent arterioles supplying each glomerulus. Efferent arterioles emerge from the glomerulus and form a dense peritubular capillary network. Venous capillaries drain into interlobular, arcuate and interlobar veins and then into the renal veins. The renal veins drain into the inferior vena cava that lies to the right of the vertebral column. Therefore, the left renal vein must be twice as long as the right one.
Nerve supply
The kidneys are supplied by the autonomic nervous system. There is a rich supply of sympathetic fibres and a few parasympathetic fibres. These fibres supply the smooth muscle of the arterioles and the juxtaglomerular apparatus. Stimulation of these nerves causes vasoconstriction, a reduced renal blood flow, a reduced glomerular filtration rate (GFR) and the release of renin from the juxtaglomerular apparatus. The kidneys also have some sensory nerve fibres that allow the sensation of pain to be perceived. These fibres are stimulated by distension of the renal capsule in such situations as bleeding, inflammation or obstruction by renal calculi. Ischaemia may also cause pain.
Renal function
The production of urine
In an adult about 180 litres of plasma are filtered every day and 99% of the filtrate is reabsorbed by the nephrons. This results in the production of about 1.5 litres of urine per day. Fluid intake, diet and extrarenal fluid losses will affect the amount of urine produced (Sherwood 2006). Glomerular filtration is the first step in urine production. Prior to describing the physiology of glomerular filtration, some concepts to facilitate understanding will be briefly outlined.
Electrolytes
These substances are solutes that are electrically charged and dissociate into their constituent ions when placed in solution. Electrolytes are polarised into those carrying a positive charge ( cations) and those carrying a negative charge ( anions). They are located in both extracellular fluid (ECF) and intracellular fluid (ICF). In ECF, sodium is the cation (Na +) and chloride is the main anion (Cl −). In ICF, potassium is the cation (K +) and protein the anion. Electrolytes are measured in milliequivalents per litre (mEq/L), which is the number of electrical charges per litre.
Diffusion
Diffusion is the movement of a solute molecule down a concentration gradient across a permeable membrane. This movement depends on the electrical potential across the membrane, the particle size, lipid solubility and water solubility.
Osmosis
Osmosis is the movement of water down a concentration gradient across a semipermeable membrane from a high water content to a lower one. The membrane must be more permeable to water than to the solutes and there must be a greater concentration of solutes in the destination solution for water to move easily. Osmosis is directly related to hydrostatic pressure and solute concentration but not to particle size. For example, in the plasma the protein albumin is smaller but more concentrated than the protein globulin; therefore albumin exerts the greater osmotic force for drawing fluid back from the ECF into the intravascular compartment.
Osmolality is the concentration of molecules per weight of water, measured in milliosmoles/kilogram. Osmolarity is the concentration of molecules in water, measured in millosmoles/litre of water. The two terms are often used interchangeably.
Hydrostatic pressure
Hydrostatic pressure is the mechanical force of water pushing against cell membranes. In the vascular system it is generated by the blood pressure. In the capillaries a hydrostatic pressure of 25 mmHg is sufficient to push water across the capillary membrane into the extracellular space. It is partly balanced by osmotic pressure. The excess water moves into the lymph system.
The amount of hydrostatic pressure needed to oppose the osmotic pressure of the solution depends on the type and thickness of the plasma membrane, size of the molecules, concentration of the molecules on the gradient and solubility of the molecules. An example would be the movement of water in the glomerulus of the kidney.
Tonicity is the effective osmolality of a solution. Solutions can be: isotonic, with the same concentration of particles as the body fluids; hypotonic, with less concentration of particles (will cause water to be pulled into the cells by osmosis); or hypertonic, with more concentration of particles (will cause water to be pulled out of the cells).
Oncotic pressure is the overall osmotic effect of the plasma proteins, sometimes called colloid osmotic pressure.
pH and acid–base balance
The pH is a measure of the hydrogen ion concentration [H +]. It is the negative logarithm of the hydrogen ions in solution on a scale of 1–14. This means that from one pH unit to the next there is a 10-fold change in hydrogen ion concentration. It is negative because as hydrogen decreases, the pH value increases. Low pH values with more hydrogen ions result in an acid solution and high pH values with a low hydrogen ion concentration result in an alkaline solution. A pH of 7 is neutral and most body fluids, with the exception of acid gastric juices (pH 1–3) and urine (pH 5–6), are just alkaline with a pH between 7 and 8. Many pathological conditions disturb the acid–base balance.
Glomerular filtration
Filtration is a largely passive, non-selective process in which fluids and solutes are forced through a membrane (i.e. filtrate) by hydrostatic pressure (Marieb & Hoehn 2008




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


