15
CHAPTER OUTLINE
■ DEFINITION (DRUGS IN THIS CLASS)
■ SUBSTANCES INCLUDED IN THIS CLASS
■ FORMULATIONS, METHODS OF USE, AND ABUSE
DEFINITION (DRUGS IN THIS CLASS)
Several heterogeneous chemicals are antagonists of the N-methyl-D-aspartate (NMDA) receptor subtype of the major excitatory neurotransmitter, glutamic acid, in the brain. Termed dissociatives, these substances can be distinguished pharmacologically and clinically from true hallucinogens (see Chapter 14). A simplified view suggests that dissociatives and hallucinogens share common features; however, hallucinogens affect primarily 5HT2A receptors instead of NMDA receptors. Hallucinogens are associated with a different 5HT2A-associated clinical syndrome of intoxication (whereby dissociation or impaired reality testing is less typically involved and visual hallucinations are more commonly involved). Dissociatives include various arylcyclohexylamines (of which phencyclidine [PCP] and ketamine are best known), dizocilpine (MK-80l), dextromethorphan (DXM), and the gaseous anesthetic, nitrous oxide. Ketamine and nitrous oxide are used clinically in animals and humans as general anesthetics, usually in combination with other agents. DXM is used clinically as an antitussive in more than 100 over-the-counter cough preparations.
Most of the known NMDA antagonists are drugs of abuse when used in subanesthetic doses/concentrations. Subanesthetic doses of PCP and ketamine induce a psychotomimetic state that resembles many but not all of the signs and symptoms of schizophrenia (1). DXM in large doses is readily metabolized to dextrorphan (DXO), a significant NMDA antagonist pharmacodynamically akin to PCP and ketamine (2). Nitrous oxide or “laughing gas” is not typically classified as a dissociative (more commonly considered an inhalant); however, given its NMDA antagonist and dissociative-like clinical effect, it merits inclusion in this chapter.
Dissociatives share the clinical effect of causing a dissociative state of intoxication that is desired by the user. The pharmacology of hallucinogens and other drugs capable of producing psychosis is discussed in their respective chapters in this section. The specific treatment of dissociative intoxication and withdrawal states is discussed in Section 6 of this book.
SUBSTANCES INCLUDED IN THIS CLASS
The chemical structures of abused arylcyclohexylamines are shown in Figure 15-1. PCP and ketamine are the principal abused illicit compounds. DXM is the principal abused over-the-counter compound. Ketamine (Ketalar, Ketalin, Keta, Calypsol, etc.) is a racemic mixture of D- and L-isomers. The (S)-isomer is more potent and is claimed to have less dysphoric effects. Other members of this class that are less commonly abused include cyclohexamine (N-ethyl-1-phenylcyclohexylamine, CI, 400), 1-(1-2-thienylcyclohexyl) piperidine, 1-(1-phenylcyclohexyl) pyrrolidine, and 4-methyl pip PCP (1-(phenylcyclohexyl)-4-methylpiperidine). Dextromethorphan (DM, DXM, D-3-methoxy- N-methylmorphinan) is the D-isomer of a codeine analog, methorphan. In contrast to the L-isomer, which is an opioid analgesic, DXM is not. The PCP-derived designer drug N-(1-phenylcyclohexyl)-3-methoxypropanamine and the ketamine analog methoxetamine should also be included in this class.
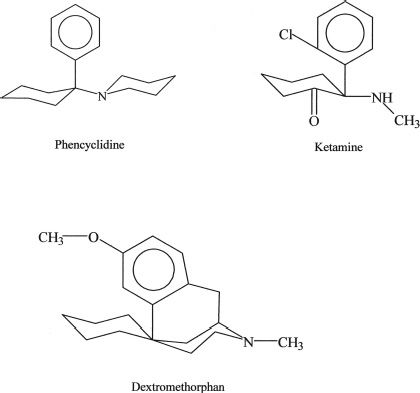
FIGURE 15-1 Chemical structures of major abused dissociative drugs.
FORMULATIONS, METHODS OF USE, AND ABUSE
U.S. Food and Drug Administration-approved Formulations
Ketamine (Ketalar) is available as a sterile solution for use in general anesthesia in both animals and humans as a U.S. Controlled Substances Act Schedule III substance. It has also been used for prehospital analgesia and anesthesia (3) and conscious sedation (4). It is used most often in children, who appear less susceptible than adults to emergent delirium (5). In medical settings, it is injected intravenously or intramuscularly in doses of 0.1 to 1.0 mg/kg, depending on its clinical use. However, it is also effective when insufflated, smoked, or taken orally. It is abused by various routes in different doses. Doses of ketamine as large as 900 to 1,000 mg given intravenously or intramuscularly are lethal.
PCP is no longer available as a medical commercial preparation approved by the U.S. Food and Drug Administration (FDA). It is available in many illicit preparations in various forms and is used in a wide range of doses. It has many of the pharmacologic effects of ketamine but is more potent, longer acting, and more likely to produce seizures. Doses of only 120 mg of PCP may cause death. It has been used as a general anesthetic in animals. It is prepared illegally in various forms: powder, tablets, and liquid (salt in water, base in ether). The latter are typically sprayed onto plant leaves such as ginger, marijuana, mint, oregano, or parsley and then smoked. PCP and some of its congeners are Schedule I substances.
Legal DXM preparations are administered orally. They are usually available as DXM hydrobromide and less commonly as DXM polistirex. Capsules, tablets, lozenges, or solutions of DXM hydrobromide are available alone or in combination with many other substances as cough, cold, and flu relief preparations. The usual antitussive dosage for adults is 10 to 20 mg every 4 hours or 30 mg every 6 to 8 hours, not to exceed 120 mg daily. Extended release forms are given as 60 mg twice daily. Larger doses of DXM are abused for their mental effects. DXM has a low toxicity and high therapeutic index (6). Death is unlikely even at very high doses but has been reported. The additional ingredients in over-the-counter preparations make for additional hazards: decongestant/pseudoephedrine (causing cardiac toxicity), antihistamine/chlorpheniramine (antihistamine toxicity), pain/acetaminophen (liver toxicity), and bromides (bromide toxicity). A serotonin syndrome has also been reported (2).
HISTORICAL FEATURES
The discovery of PCP has been well documented by those involved with its therapeutic development (7). The drug was developed as an intravenous anesthetic. The unique anesthesia it produced was complicated by a prolonged emergence delirium. This quickly led to its demise as a clinically useful agent. PCP is associated with symptoms that model both the positive (delusions, hallucinations) and negative (blunted affect, ambivalence, asociality, autistic-like effects) symptoms of schizophrenia, making for perhaps one of the more useful pharmacologic models of schizophrenia (8,9). Its trade name was Sernyl or Semylan. Years later, PCP was rediscovered by the drug abuse community and has also been known as “PCP,” “angel dust,” “hog,” and “crystal” (10).
The desirable anesthetic properties of PCP were retained in the short-acting arylcyclohexylamine derivative ketamine, which produced a briefer emergence delirium. The term dissociative anesthetic was coined to emphasize that the anesthetized patient was psychologically “disconnected” from his or her environment. Ketamine subsequently was discovered by the drug abuse community, where it is known as “K,” “super K,” “special K,” and “cat Valium,” among others. Ketamine has the reputation among users as being medically safe to use because it is made and packaged by pharmaceutical companies, most often for veterinary use (11).
Arylcyclohexylamine abuse occurs primarily in large metropolitan areas. Because the drugs are easy to synthesize, they are relatively inexpensive substitutes for many street drugs. The user may not realize that he or she has used an arylcyclohexylamine because the drugs frequently are misrepresented as LSD-25, amphetamine, or synthetic marijuana. Moreover, they may be added to marijuana by the user to enhance marijuana’s desired effects.
In contrast to the arylcyclohexylamines, MK-801 (dizocilpine) was developed as an anticonvulsant (12) and subsequently was used as a brain-protective agent; however, it was discarded because of its PCP-like effects (13). Clinical trials of MK-801 have been extremely limited, and the results are not publicly available. Very little is known of its properties in humans.
The history of DXM begins with the synthesis of race-methorphan (deoxydihydrothebaiodine) or methorphan (Dromoran) and its patenting by Hoffmann-LaRoche in 1954 as an opioid analgesic. After the D- and L -isomers were isolated, it was discovered that the D-isomer was antitussive and had less analgesic and narcotic-like properties. DXM is nearly equal to codeine as an antitussive. However, unlike codeine, DXM is fairly devoid of other opioid effects such as analgesia, central nervous system depression, and respiratory suppression. Although it is metabolized to DXO, an NMDA receptor antagonist, its binding sites in the brain include more than the NMDA receptor. DXM’s mechanism in low doses as an antitussive is unknown. In doses of 300 to 1,800 mg (20 to 120 times the recommended dose), DXM produces PCP-like mental effects (14,15). However, larger doses (237 times the recommended dose) are regularly abused (16). DXM abuse has been a concern since at least the 1960s. The over-the-counter tablet form of DXM, Romilar, was replaced by a cough syrup in 1973 to reduce its recreational use. In 1990, the FDA Drug Abuse Advisory Committee assessed DXM use by teenagers and recommended against placing the drug on the Controlled Drug Schedule but requested more study of the problem (17). Although abuse of DXM began originally with abuse of liquid cough syrup (known to hamper the abuse of large doses of DXM because of the distasteful nature of cough syrup), more convenient consumer products have since been developed, including both high-dose (30 mg) tablets as well as high-dose gel capsules, which are preferred by those who abuse DXM. Acid-base extraction techniques have been developed by users to “free base” the DXM alone (18). DXM has become popular, particularly among children and adolescents, owing to this population incorrectly perceiving DXM as a “SMART” drug to abuse; that is, they perceive DXM abuse as without stigma, not costing much money to procure, having easy access at local stores, being devoid of medical risks, and not included in routine employment or home-based drug testing (19). The FDA continues to express its concern over the abuse of DXM, in particular the purified powder form purchased via the Internet (20). Slang terms for the cough medicine preparations are “dex,” “robo,” “skittles” (owing to some tablets appearing similar to red Skittles candies), “tussin,” and “triple Cs.” Recreational use of DXM is described as “dexing,” “roboing,” and “robotripping” (referring to the popular DXM cough syrup Robitussin®).
Nitrous oxide has been known for more than 225 years. It is widely used today in anesthesia. In addition, its recreational use as “laughing gas” has been well described since it first was discovered. Ketamine and nitrous oxide still are medically used in humans as anesthetic agents. Ketamine is used in circumstances in which other anesthetic agents are relatively contraindicated. In contrast, nitrous oxide is widely used today as part of the mixture of anesthetics used to achieve “balanced anesthesia.”
The desirable “brain-protective” properties of NMDA antagonists, including DXM, have been pursued by the pharmaceutical industry in developing relatively weak derivatives of amantadine and other so-called sigma receptor agonists and antagonists (21). For example, amantadine is an antiviral agent used in the prophylaxis and therapy of influenza A and to treat parkinsonism. Patients with Parkinson disease who took amantadine reported that it improved their motor symptoms. The mechanism of action of amantadine is unclear, but may include dopamine release or blockade of its reuptake and possible muscarinic anti-cholinergic action. Amantadine and a related compound, memantine, are NMDA receptor antagonists (22), but are relatively weak and do not appear to be abused.
EPIDEMIOLOGY
PCP abuse may be more a problem in large cities like Washington, DC, Philadelphia, Miami, and Los Angeles than in the rest of the United States (23). Ketamine is typically used with other drugs (24); however, sole use of ketamine has been reported (11) and may be increasing, particularly in Asian countries. Although ketamine has often been self-administered by insufflation, there is an emerging problem in youth of injecting ketamine. Such youth are more likely to engage in multiple injections, shared bottles of ketamine, and use of syringes obtained from secondary sources-practices that increase risk for hepatitis C, HIV, and other infectious diseases (25).
DXM is considered one of the most commonly abused over-the-counter medications in the United States and was first included in the Monitoring the Future epidemiologic surveys in 2006. The proportion of US students who reported in 2006 having used DXM during the prior year for the expressed purpose of “getting high” was 4%, 5%, and 7% in grades 8, 10, and 12, respectively (26). Rates in 2011 were similar, at 3%, 6%, and 5%, respectively (27). Poison Control Center data from the first half of the 2000 to 2010 decade reflected increasing DXM abuse, particularly among adolescents (28). For example, cases of DXM abuse reported to the California Poison Control System (28) increased 10-fold in all age groups and 15-fold in adolescents between 1999 and 2004. Similar trends were seen in national databases. Approximately 75% of California cases were aged 9 to 17 years. The highest frequency of abuse was in 15- to 16-year-olds. The most commonly abused DXM product was Coricidin HBP Cough & Cold Tablets. The extent of DXM abuse is likely far greater than what has been reported, because only the most severe cases are reported to poison control databases, and nearly all routine drug screening kits still do not screen or test for DXM or DXO. Studies of DXM in blood samples of suspected impaired drivers in the state of Wisconsin between 1999 and 2005 also supported an increasing prevalence of DXM-positive drivers, with a mean concentration of 207 ng/mL—compared with an expected therapeutic concentration range of 0.5 to 5.9 ng/mL (the highest concentrations being in males aged 16 to 20 years) (29). Intentional abuse of Coricidin products reported to the Illinois poison center occurred primarily among adolescents and was associated with significant short-term clinical effects and $353,314 in hospital charges annually (2001–2006) (30). The national upward trend in DXM abuse in the first half of the decade (2000–2005) has since peaked at 17.6 calls per million population in 2006 and plateaued at 15.7 per million in 2010 (31).
PHARMACOKINETICS
The pharmacokinetics of PCP in humans have never been well studied with psychoactive doses using modern methods (6). Blood PCP concentrations from 7 to 240 ng/mL (mean, 75) were found in arrested persons intoxicated in public or driving under its influence. The blood/plasma concentration ratio is 1. The plasma half-life (t½) of PCP has been reported to vary from 7 to 46 hours, suggesting the influence of dose and/or multiphase elimination processes. A terminal elimination phase (gamma) t½ of 1 to 4 days has been reported in cases of severe PCP poisoning (6). PCP is biotransformed in the liver to several metabolites and excreted in the urine as both free and glucuronide conjugates. Acidification of the urine increases its renal clearance because PCP is a base. However, this maneuver is no longer recommended clinically because of the risk of increasing urinary myoglobin precipitation (32).
Ketamine’s greater lipophilicity than PCP’s accounts for its rapid onset, short anesthetic duration of action, and shorter period of emergence delirium. Plasma concentrations of ketamine vary widely depending on the dose, route, and time elapsed since administration. Anesthetic doses produce plasma or serum concentrations of 1 to 6.3 ?g/mL.
Nonanesthetic psychoactive blood concentrations of ketamine are in the low nanograms per milliliter range (100 to 400). Ketamine follows a three-phase plasma pharmacokinetic model when given intravenously (6,33). There is a brief initial (alpha) phase with t½ of about 7 minutes because of rapid redistribution, followed by a longer elimination (beta) phase with t½ of 3 to 4 hours. As used in general anesthesia, an intravenous dose of 2.0 mg/kg produces rapid induction. This dose produces an onset in 30 seconds, with the coma lasting for 8 to 10 minutes. The intramuscular injection of ketamine has a latency of 3 to 5 minutes and a duration of 10 to 20 minutes or more, depending on the dose administered.
DXM is readily absorbed from the gut. Peak serum levels are reached at 2 to 3 hours for immediate release and 6 hours for sustained release preparations. DXO levels peak at 1.6 to 7 hours (34). Humans have a genetic polymorphism for the biotransformation of DXM (6). Rapid metabolizers have a plasma elimination t½ of about 3.4 hours, and slow metabolizers may have t½ exceeding 24 hours. Slow metabolizers of DXM represent about 10% to 15% of the population. Both O– and N-demethylation of DXO occur, with the N-demethylated version also having antitussive effects. Subsequent biotransformation results in various less active compounds. Phenotypic “slow” metabolizers of DXM report fewer intoxication effects than normal subjects (35). Thus, clinically slow metabolizers might be at higher risk for developing DXM dependence/addiction (2).
PHARMACODYNAMICS
Depending on the dose and specific arylcyclohexylamine ingested, patients who have taken PCP or ketamine present with widely different neurologic and psychiatric signs and symptoms. These signs and symptoms can be generally subdivided into three major clinical pictures: (a) confusion, delirium, and psychosis; (b) semicoma and coma; and (c) coma with seizures. Patients may become progressively more obtunded and eventually comatose, or the reverse, with the patient emerging from coma and showing emergence delirium. Table 15-1 lists the various signs and symptoms of PCP intoxication at different intravenous doses. Figure 15-2 shows the correlation of the molecular target sites with doses, concentrations, and clinical effects (36). Most PCP abusers do not grossly overdose themselves to the point of semicoma and coma. Hence, most patients intoxicated with PCP show a clinical picture of confusion, delirium, and psychosis. Rats show marked behavioral sensitization to both PCP and MK-801, with asymmetric cross-sensitization (37–39). The significance of this phenomenon for humans is unknown. Whether individuals who abuse PCP or ketamine show enhanced psychotomimetic effects over time is also unknown. Tolerance occurs with PCP and to a greater degree with continuous dosing (40). Animal models show severe withdrawal after cessation of PCP exposure: vocalizations, bruxism, oculomotor hyperactivity, diarrhea, piloerection, somnolence, tremor, and seizures (41). However, human dissociative withdrawal is not formally recognized by the Diagnostic and Statistical Manual, and human evidence remains limited (42,43).
TABLE 15-1 DOSE-RELATED EFFECTS OF PCP IN HEALTHY SUBJECTS
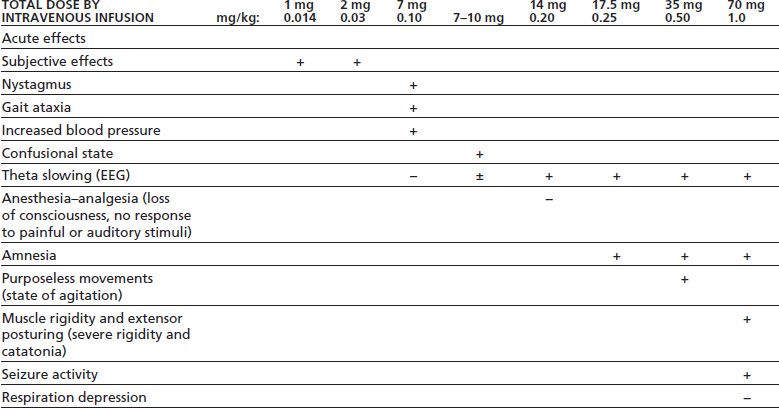
Data summarized by Burns & Lerner in Domino EF, ed. Phencyclidine: historical and current perspectives. Ann Arbor, MI: NPP Books, 1981:450.
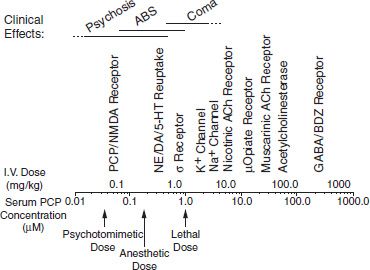
FIGURE 15-2 PCP doses, serum concentrations, molecular target sites, and clinical effects.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


