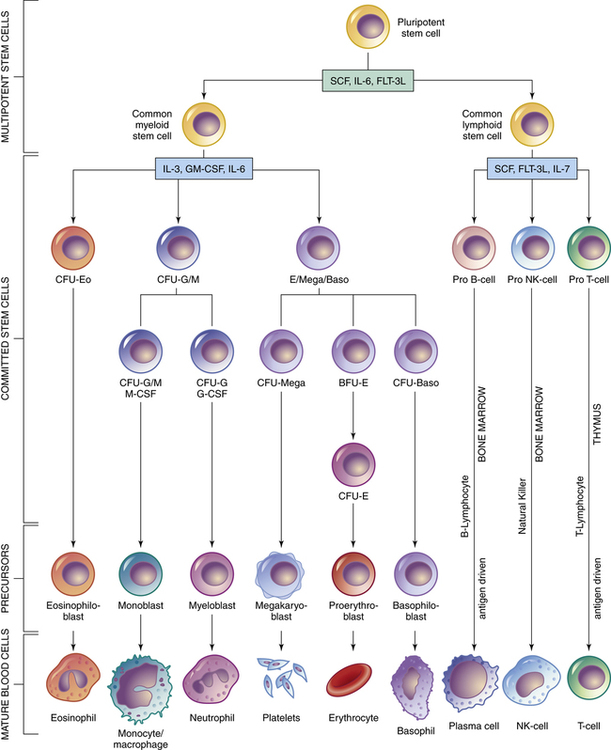
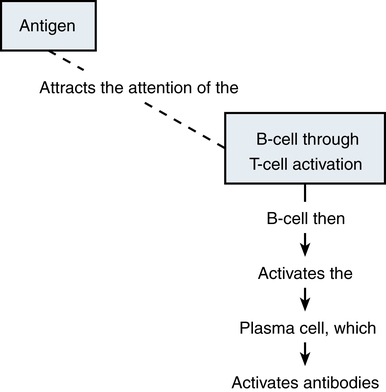
Chapter 70 IMMUNIZATION AND BIOLOGIC AGENTS COMMONLY USED IN PRIMARY CARE Organs of the immune system consist of primary and secondary organs. Primary organs are responsible for the development and storage of lymphocytes. The bone marrow and the thymus gland are the primary organs of the immune system. Secondary organs include lymph nodes, spleen, and Peyer’s patches. These secondary organs of the immune system entrap foreign substances, produce antibodies, and stimulate T-cell production, all with the main objective of destroying the antigen. All WBCs originate from a stem cell in the bone marrow. Stem cells first differentiate into myeloid and lymphoid cells. Myeloid cells differentiate into polymorphonuclear (PMN) leukocytes and into monocytes/macrophages. Lymphoid cells differentiate into B- and T-lymphocytes (Figure 70-1). Two types of nonspecific WBCs and two types of specific WBCs have been identified. • Neutrophils contain large granules. These granules degranulate when they come in contact with antigens and release enzymes that destroy foreign substances and can injure surrounding tissue. Debris from this destructive action produces an exudate/pus. Enzymes that are secreted from these granules are known as chemotaxic factors; they include leukotrienes, vasoactive kinins, and toxic metabolites. • Eosinophils are very similar to neutrophils. They contain granules and engage in the process of phagocytosis. They seem to congregate in the respiratory and gastrointestinal tracts. They are especially prominent during allergic reactions and parasitic infections, and they carry certain enzymes that neutralize chemicals responsible for allergic responses. They release potent chemotaxic factors that cause inflammation, bronchospasm, and tissue damage. • Basophils also contain granules that produce histamine and heparin, which play a role in the immune response. The basophil is not a strong structure, and it is easily damaged, which causes the granules to release histamine and heparin. Vasospasm, increased vascular permeability, and increased inflammation are the major effects seen when this occurs. This reaction increases the severity of allergic responses. • Mast cells, the guardians of the immune system, are found in cutaneous and mucosal tissue. They can immediately recognize invasive non-self (foreign) antigens without the aid of macrophages or lymphocytes. They are the effectors of immediate hypersensitivity reactions and contain most of the body’s IgE. When this IgE and an antigen meet, there is immediate degranulation and release of histamine, prostaglandin, and leukotrienes, as well as arachidonic acid metabolism, which potentiates the hypersensitivity response. • The B-lymphocytes are those cells that produce antibodies. They undergo specific differentiation when exposed to an antigen and become plasma cells that are the major secretors of antibodies (Figure 70-2). The major function of these antibodies is to destroy a specific antigen and remove it from the body. In response to a specific antigen, a single antibody is produced (each antigen has a specific antibody). The antibodies are grouped into five different classes known as immunoglobulins. These classes contain formed chains of immunoglobulins expressed on the cell surface and labeled IgG, IgA, IgM, IgE, and IgD. Table 70-1 describes the features of these antibodies. B-lymphocytes provide humoral immunity through the secretion of these immunoglobulins. TABLE 70-1 Characteristics and Functions of Immunoglobulins • T-cell lymphocytes make up 65% to 80% of all lymphocytes in the blood. Three different types of T-cells are known: helper, suppressor, and cytotoxic. Helper cells aid in initiation of the immune response by helping B-cells synthesize antibodies for action. Suppressor cells help keep B-cell antibody production in check. They hold back the immune response or restrict antibody production because, if left unchecked, these B-cells can do more harm than good. Cytotoxic cells, or killer cells, circulate to kill cells not recognized as self cells (such as tumor cells). Activation of these cells occurs through interaction of antigens with macrophages. Through secretion of the special products produced by macrophages, T-cell proliferation occurs. These activated T-cells release substances known as lymphokines that influence the growth of other cells necessary for body defense, thus amplifying the immune reaction (Figure 70-3). Three plasma protein systems are located in the plasma of blood, not inside a cell. These include the complement, clotting, and kinin systems. Each initiates a cascade of reactions, ending with potent biochemical mediators of the inflammatory response. The complement system is a nonspecific mediator of inflammation that is potent against bacterial infection. IgG or IgM usually initiates the cascade by forming an immune complex. The kinin system begins with bradykinin, which causes dilation of vessels, acts with prostaglandins to induce pain and increase vascular permeability, and is important in the prolonged phase of inflammation. Platelets stop bleeding and release serotonin, which has vascular effects similar to histamine. The clotting system is discussed in Chapter 25. Cytokines are glycoproteins, which are chemical messengers that modulate the immune response. Prostaglandins cause increased vascular permeability and neutrophil chemotaxis (movement), and they induce pain. Increased vascular permeability allows diffusion of large molecule inflammatory substances across cell walls into the site of inflammation. Prostaglandins are made within the mast cell from arachidonic acid through the action of the enzyme cyclooxygenase (COX) and are classified into groups according to their structure. Prostaglandins E1 and E2 are active in the inflammatory response. Aspirin and NSAIDs act to block the enzyme COX from producing prostaglandins, thereby inhibiting inflammation (see Chapter 34 and Figure 34-2). • A foreign substance (the antigen) invades. • A macrophage engulfs it and presents it on its cell surface. • The expressed antigen stimulates T-cell activity. • T-helper cells are activated to enhance B-cell differentiation into plasma cells and the production of antibodies/immunoglobulin (e.g., IgG, IgM, IgA, IgE, IgD). • Proliferation of B- and T-cells produces clones with a memory that enables them to recognize a returning invader. This memory produces a more potent and rapid immune response should the invader return. Active immunization is accomplished with three different types of agents: 1. Inactivated vaccines (killed agents): Most bacterial vaccines, and some viral, involve the use of inactivated agents. These agents are not capable of replicating within the host and thus present little risk to the recipient. Maintenance of lifelong immunity requires the administration of multiple doses. Mucosal protection after the use of killed vaccines is less than with the use of live vaccines. Thus, local infection or colonization with the agent can occur, along with potential for transmission, although systemic disease is prevented. 2. Live vaccines (attenuated): Most viral vaccines involve the use of live virus that has been chemically changed to decrease its virulence. Active infection, with replication of the virus, occurs in the host following administration of the product, although few adverse effects occur. This route generally produces a superior response, including mucosal immunity, and does not require the use of multiple doses. 3. Active immunization: This may be accomplished with use of a modified product of an organism, such as a toxoid, which consists of modified bacterial toxins and retains the ability to stimulate antibody formation but is nontoxic. This route of immunization is used against diphtheria and tetanus. Maintenance of protective titers of antitoxin requires periodic administration of booster doses of toxoid. Passive immunity occurs when antibodies that one acquired from a human or an animal (with acquired immunity to a specific organism) are given to people who do not have immunity to the organism. Newborn infants achieve this naturally from their mothers through the placenta and through breastfeeding. It can also be achieved through injections of gamma globulins (for hepatitis protection) or antisera or antitoxins. This process temporarily provides the same protection as that given to a person who has achieved active acquired immunity. These antibodies naturally break down and are eliminated from the body. See Table 70-1 for a summary of the characteristics and function of immunoglobulins. All immunizations contain several different components, such as the following: • Immunizing agent: This active component of the product may be a killed or attenuated vaccine or a toxoid. • Suspending fluid: This may be either sterile water or saline or a complex tissue-culture fluid. It may contain proteins derived from the medium in which the vaccine was produced, such as egg antigen in inactivated influenza vaccine. Individuals who experience anaphylactic reactions to egg may be allergic to influenza vaccine; studies of MMR vaccine have demonstrated that egg-allergic children may be safely vaccinated. • Preservatives: Trace amounts of preservatives, stabilizers, or antibiotics often are added to prevent bacterial overgrowth in multiuse vials of vaccines. Individuals may have allergic reactions to products such as neomycin that may be present in minute amounts. Thimerosal, a mercury-containing organic compound widely used as a preservative, was removed from or was reduced to trace amounts in all vaccines with the exception of inactivated influenza vaccine in 2001 because of concerns about potential neurodevelopmental pathology in infants who may receive multiple vaccines that contain thimerosal and may be unable to adequately clear this product because of hepatic immaturity (see http://www.fda.gov/cber/vaccine/thimerosal.htm). In 2004, the Institute of Medicine released a report that rejected a causal link between vaccines and developmental disorders, including autism, in children. The mercury levels found in the urine of children with autism were not greater than the levels in those without the condition, according to a U.K. study. Researchers noted that other heavy metal concentrations also were the same among children with autism, children from the general population, children without the condition but who had siblings with autism, and children who attended special education schools. The FDA is continuing its efforts to reduce the exposure of infants, children, and pregnant women to mercury from various sources. • Adjuvants: This is often an aluminum-based compound that is added to enhance the immunogenicity of an agent and prolong its stimulatory effects. This is necessary for some inactivated vaccines and for toxoids. Figure 70-4 shows the recommended childhood immunizations generally used in primary care. • Recommended childhood, adolescent, and adult immunization schedules: United States, 2012 • Centers for Disease Control Advisory Committee on Immunization Practices (ACIP) All are available at http://www.cdc.gov/vaccines/recs/schedules/default.htm. • ACIP: Recommended Adult Immunization Schedule: United States, 2012, MMWR 61(04);1-7, February 3, 2012 (Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6104a9.htm) • ACIP: Recommended Immunization Schedules for Persons Aged 0 Through 18 Years: United States, 2012, MMWR 61(05);1-4, February 10, 2012 (Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6105a5.htm) • General principles for vaccine administration: • The Recommended Childhood and Adolescent Immunization Schedule and the Recommended Adult Immunization Schedule are revised annually. Health care providers should ensure that they are following the most up-to-date schedules, which are available on the CDC website. • Vaccination providers should adhere as closely as possible to recommended vaccination schedules. Longer-than-recommended intervals between doses do not reduce final antibody concentrations, although protection might not be attained until all doses have been administered. With the exception of oral typhoid vaccine, an interruption in the vaccination schedule does not require restarting the entire series of a vaccine or toxoid or addition of extra doses. • Health care providers should simultaneously administer all vaccines for which a person is eligible because simultaneous administration increases the probability that an individual will be vaccinated fully at the appropriate age. Administration of each preparation at a different anatomic site is desirable. Simultaneous administration of the most widely used live and inactivated vaccines has produced seroconversion rates and rates for adverse reactions similar to those observed when the vaccines are administered separately. An inactivated vaccine can be administered simultaneously or at any time before or after a different inactivated vaccine or live vaccine. Live vaccines also may be administered simultaneously. However, live vaccines such as measles, mumps, and rubella should not be administered after receipt of an antibody-containing product such as immunoglobulin until the passive antibody response to the product has degraded. This process is dependent on dose and specific product. Exceptions to this rule are yellow fever vaccine, oral typhoid vaccine, and live-attenuated influenza vaccine, which may be administered after immunoglobulin. • Use of combination vaccines can reduce the number of injections required at a visit. Licensed combination vaccines can be used whenever any components of the combination are indicated, and its other components are not contraindicated. • Vaccines made by different manufacturers are generally interchangeable. Although it is preferable to complete a series with a single product, vaccination should not be deferred because the brand used for previous doses is not available or is unknown • Persons without documentation of vaccine receipt should be considered nonimmunized if a reasonable effort to locate records is unsuccessful. These individuals should be started on the age-appropriate vaccination schedule. Serologic testing for immunity is an alternative to vaccination for certain antigens (e.g., measles, rubella, hepatitis A, tetanus). Guidelines concerning the use of immunizations are one of the most carefully studied areas of pharmacology. Joint guidelines for children are promulgated by the ACIP and the American Academy of Pediatrics (AAP). Guidelines are revised yearly as new products are introduced and recommendations for use of older products change; these guidelines determine the standard of care that all primary care providers are expected to provide. With the increase in the immunocompromised population and the increasing numbers of immigrants living in the United States who require catch-up vaccination, the greatest challenge for primary care providers is to master the exceptions to the recommended schedule. Figure 70-4 provides the recommendations for pediatric immunizations from the ACIP and the AAP. The latest updates are available on the CDC website. Some of the important exceptions are summarized as follows: 1. In compliance with recommendations for all individuals, it is particularly important that all health care workers be immunized against hepatitis B. In addition, health care workers in emergency department settings and on emergency response teams, such as emergency medical technicians and paramedics, may elect to be vaccinated against smallpox. Specific recommendations for the use of smallpox vaccine are discussed later in this chapter. 2. Travelers to many underdeveloped nations may require specific immunizations, depending on where they are traveling. The CDC publishes information on vaccine recommendations for travel that can be found on the CDC’s Traveler’s Health website. The CDC Health Information for International Travel, commonly referred to as the Yellow Book, is updated every 2 years and is the authoritative source of U.S. government recommendations for immunizations and prophylaxis for foreign travel. 3. The CDC’s Guide to Vaccine Contraindications and Precautions was last updated in 2009 and can be found on the CDC website. 4. Immunosuppressed individuals, including those with HIV, individuals receiving chemotherapy, and those on corticosteroids, will require modifications of the recommended immunization schedule. Where possible, efforts should be made to appropriately vaccinate individuals prior to beginning regimens such as chemotherapy. • Minor upper respiratory infection or gastroenteritis, with or without fever, is not an appropriate indication for withholding a scheduled vaccine dose. • Concurrent administration of an antibiotic is not a contraindication to immunization. • In most cases, preterm infants should be immunized at the usual recommended chronologic age and with the recommended dose. Premature infants weighing under 2000 grams should not receive Hepb until 1 month of age or prior to hospital discharge. • Pregnancy is a contraindication to administration of live vaccines. However, pregnancy in a household member is not a reason to withhold vaccine from a child or adult. • Women who are breastfeeding may be immunized with all products, with the exception of smallpox and yellow fever vaccines. While other vaccines, including hepatitis A and polysaccharide pneumococcus, have not been specifically studied in breastfeeding women, no evidence suggests that trace amounts of a vaccine present in breast milk are harmful to infants. • A lapse in the recommended immunization schedule does not require that the entire series be restarted. Needed doses should be given at the first opportunity as though the usual interval had occurred. • Half-doses of vaccine are never indicated in individuals who had a significant reaction to a previous dose. In addition, reduced or divided doses are not recommended for preterm or low birth weight infants. • Family history of an adverse event with an immunizing agent, family history of seizures, and family history of sudden infant death are not appropriate indications for withholding recommended immunizations. • Soreness, redness, or swelling in the area of the immunization or fever lower than 40.5°C (105°F) following a previous vaccine is not an indication to withhold subsequent doses. • Early vaccinations against pertussis, Haemophilus influenzae type b, and measles-mumps-rubella in babies were not linked to the risk of developing celiac disease. All immunizing agents have specific contraindications that will be discussed separately. The legislation requires health care providers who administer vaccines to keep permanent records of all immunizations given, along with specific information about the manufacturer of the product and the lot number of all doses. In addition, the provider is required to report occurrences of events suspected to be the result of vaccine by using a mechanism titled the Vaccine Adverse Event Reporting System (VAERS). Reports may be made online at http://vaers.hhs.gov. The specifics of reportable events for each agent are discussed separately. In addition, this act established a Vaccine Injury Compensation Table that determined the injuries, disabilities, and conditions for which compensation may be made.
The Immune System and Immunizations
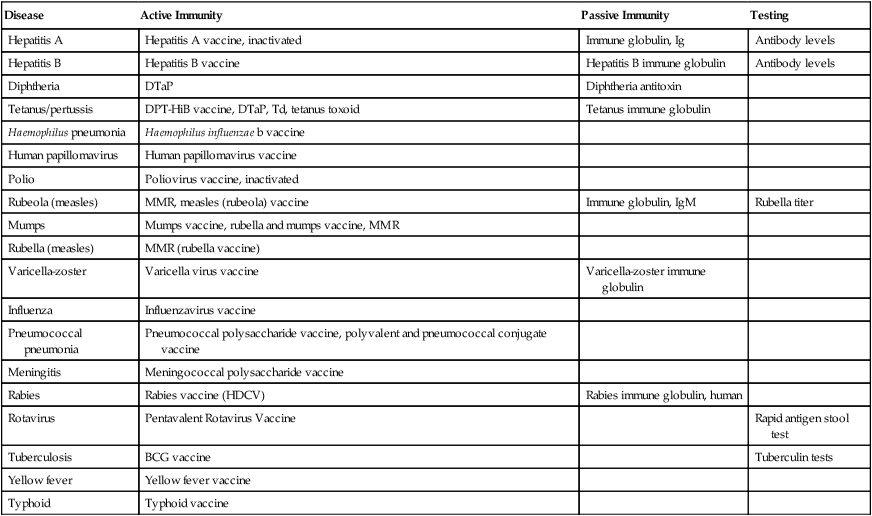
Disease
Active Immunity
Passive Immunity
Testing
Hepatitis A
Hepatitis A vaccine, inactivated
Immune globulin, Ig
Antibody levels
Hepatitis B
Hepatitis B vaccine
Hepatitis B immune globulin
Antibody levels
Diphtheria
DTaP
Diphtheria antitoxin
Tetanus/pertussis
DPT-HiB vaccine, DTaP, Td, tetanus toxoid
Tetanus immune globulin
Haemophilus pneumonia
Haemophilus influenzae b vaccine
Human papillomavirus
Human papillomavirus vaccine
Polio
Poliovirus vaccine, inactivated
Rubeola (measles)
MMR, measles (rubeola) vaccine
Immune globulin, IgM
Rubella titer
Mumps
Mumps vaccine, rubella and mumps vaccine, MMR
Rubella (measles)
MMR (rubella vaccine)
Varicella-zoster
Varicella virus vaccine
Varicella-zoster immune globulin
Influenza
Influenzavirus vaccine
Pneumococcal pneumonia
Pneumococcal polysaccharide vaccine, polyvalent and pneumococcal conjugate vaccine
Meningitis
Meningococcal polysaccharide vaccine
Rabies
Rabies vaccine (HDCV)
Rabies immune globulin, human
Rotavirus
Pentavalent Rotavirus Vaccine
Rapid antigen stool test
Tuberculosis
BCG vaccine
Tuberculin tests
Yellow fever
Yellow fever vaccine
Typhoid
Typhoid vaccine
Therapeutic Overview
Anatomy and Physiology: The Immune System
Nonspecific WBCS
Specific WBCs
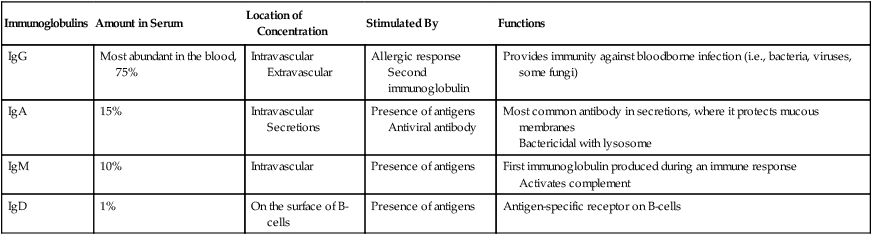
Immunoglobulins
Amount in Serum
Location of Concentration
Stimulated By
Functions
IgG
Most abundant in the blood, 75%
Intravascular
Extravascular
Allergic response
Second immunoglobulin
Provides immunity against bloodborne infection (i.e., bacteria, viruses, some fungi)
IgA
15%
Intravascular
Secretions
Presence of antigens
Antiviral antibody
Most common antibody in secretions, where it protects mucous membranes
Bactericidal with lysosome
IgM
10%
Intravascular
Presence of antigens
First immunoglobulin produced during an immune response
Activates complement
IgD
1%
On the surface of B-cells
Presence of antigens
Antigen-specific receptor on B-cells
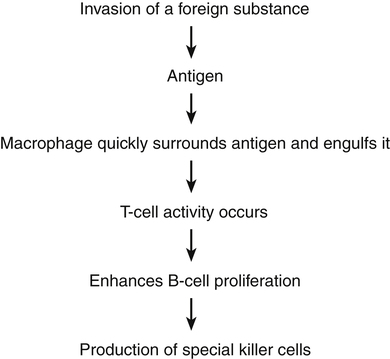
Other Immune System Components
The Nonspecific Inflammatory Response
The Specific Immune Response
Therapeutic Overview
Active Immunity
Passive Immunity

Schedules for persons aged 7 to 18 years, catch-up for persons aged 4 months to 18 years, and adult immunizations are available at http://www.cdc.gov/vaccines/recs/schedules/default.htm. From Centers for Disease Control and Prevention, National Immunization Program: Recommended childhood and adolescent immunization schedule: United States 2011. http://www.cdc.gov/vaccines/schedules/downloads/child/0-6yrs-schedule-pr.pdf.
Treatment Principles
Standardized Guidelines
Evidence-Based Recommendations
Cardinal Points of Treatment
At-Risk and Postexposure Patients
Principles of Administration of Vaccines
Notification of Risks and Benefits of the Vaccine
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree