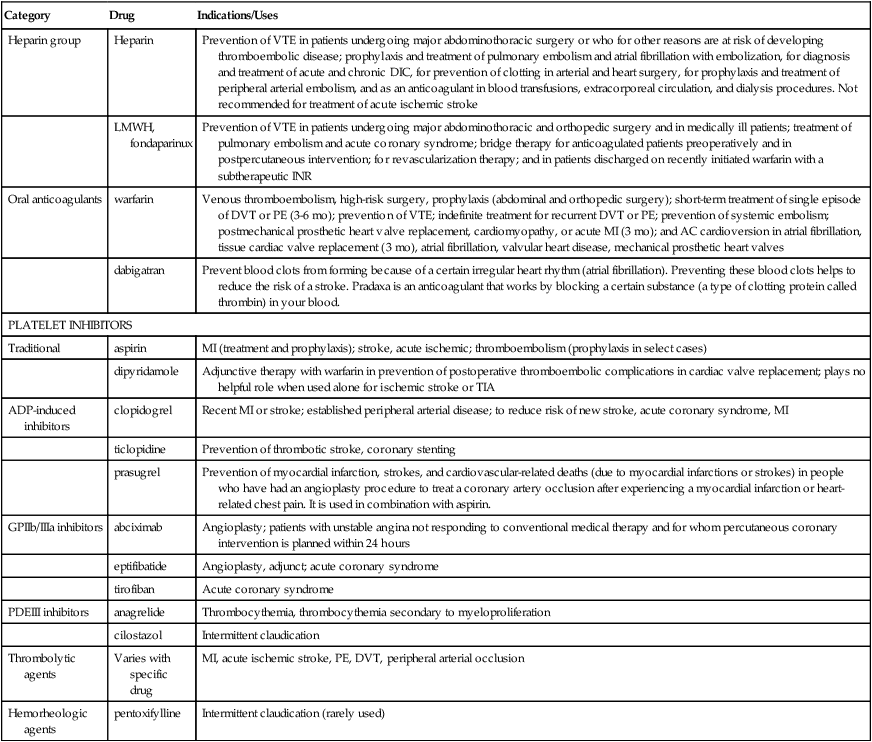
Chapter 25 Theresa Pluth Yeo and Susan E. Shirato A delicate balance must be maintained between the fluidity of the bloodstream and the ability of blood to clot quickly to prevent hemorrhage. In the coagulation system, two pathways are necessary for clotting. The intrinsic clotting pathway is initiated when the blood is exposed to a negatively charged surface such as the in vitro coagulation activators celite, kaolin, or silica. The intrinsic pathway is triggered when blood comes into contact with damaged endothelium or collagen. The extrinsic clotting pathway is triggered by exposure of tissue factor at the site of tissue injury or the addition of thromboplastin to blood. The intrinsic and extrinsic pathways merge on the activation of factor X in what is referred to as the final common pathway (Figure 25-1). Factor X then converts factor II (prothrombin) to IIa (thrombin), and factor IIa converts fibrinogen to a fibrin clot (thrombus). A number of acquired factors are associated with increased risk for a thromboembolic event (Box 25-1). Most of these factors are linked to decreased circulation, reduced mobility, or obstruction of blood flow. Many are the result of other diseases or disability or disabilities. One of the critical decisions that a clinician must make is whether it is possible to reduce or control risk factors in order to minimize the chance of thromboembolic events. Clinicians should suspect inherited risk factors, such as deficiency of anticoagulation proteins C and S, antithrombin III deficiency, factor V Leiden mutation, prothrombin G20210A mutation, and factor VIII elevations in patients displaying unusual procoagulable or prothrombotic tendencies. Hyperhomocysteinemia is found in about 5% of the population and is associated with a threefold increase in VTE. Virchow’s triad describes inherited and acquired conditions that place patients at increased risk for developing emboli; these include hypercoagulable states, endothelial injury, and circulatory stasis. • Assess personal and family history of bleeding and vascular disorders, lifestyle, and alterable risk factors such as obesity and immobility. • Check platelets, INR, a PTT, CBC with platelets, stool Hemoccult, creatinine/blood urea nitrogen, and liver function tests. The clinician may wish to test for hyperhomocysteinemia—as elevated serum levels of homocysteine have been reported for arterial and venous occlusive disease in relation to low levels of folate, vitamin B6, and vitamin B12. Heparin has no effect on existing clots; it prevents or retards formation of new thrombi. Heparin acts at multiple sites in the coagulation system and binds with antithrombin III (AT-III) at two specific sites, resulting in its anticoagulant effect. At the first heparin–AT-III binding site, factor Xa is neutralized, thereby exerting a direct effect on factor X. Factor X is responsible for initiating the final common pathway in the clotting cascade (see Figure 25-1), which ends in clot formation. The second heparin–AT-III binding occurs at the site of conversion of prothrombin to thrombin (factor IIa). With decreased thrombin available, a reduced amount of fibrin is made from fibrinogen. Standard UFH is a mixture of polysaccharide molecules that vary in average molecular weight and composition. Typically, only one third of the molecules in a standard UFH preparation contain the pentasaccharide sequence needed for antithrombin binding and anticoagulation. It is critical to understand these vitamin K–dependent clotting factors and their half-lives if warfarin is being prescribed. Warfarin is the number one drug causing adverse drug effects in hospitals, sometimes because clinicians do not understand these times. The activity of various clotting proteins (logarithmic scale) is shown in Table 25-2 as a function of time after ingestion of warfarin (10 mg/day po for 4 consecutive days) by a normal subject. Factor VII activity, to which prothrombin time is most sensitive, is the first to decrease. Full anticoagulation, however, does not occur until factors IX and X and prothrombin are sufficiently reduced. Protein C activity falls quickly, and, in some patients, a transient hypercoagulable state may ensue (e.g., coumarin necrosis) (Furie, 2000). TABLE 25-2 Approximate Half-Life of Vitamin K–Dependent Factors and Anticoagulants Compiled from Hoffman R et al, eds: Hematology: basic principles and practice, ed 3, New York, 2000, Churchill Livingstone. • The eighth edition of Antithrombotic and Thrombolytic Therapy: American College of Chest Physicians (ACCP) Evidence-Based Clinical Practice Guidelines and the 2010 Guidelines for Prevention of Stroke in Patients with Ischemic or Transient Ischemic Attack from the Stroke Council of the American Heart Association statement on primary prevention of stroke have provided important new information on the management and prevention of thromboembolic disorders, bridging therapy for those on anticoagulants during an invasive procedure, acute coronary syndromes, and ischemic stroke. Although prevention of ischemic stroke is the primary outcome of interest, the guidelines also identify strategies for the reduction of all vascular outcomes after stroke or TIA, including subsequent stroke, myocardial infarction (MI), and vascular death. Treatments for proximal DVT include the following: Thrombotic treatment in ischemic heart disease includes the following: • Beneficial: aspirin, oral anticoagulants in the absence of antiplatelet treatment, thienopyridines • Use strategies that increase thromboprophylaxis, including computer-based decision making support systems, preprinted orders, audits, and patient feedback. • Identify the drugs to be used in prevention, acute treatment, or maintenance therapy. • Begin therapy and monitor closely to achieve anticoagulation level desired. • Provide adequate patient and family education regarding diet, activity, signs for which to monitor, and the need for further laboratory monitoring. UFH is administered as a continuous intravenous infusion on an inpatient basis. For the prevention of VTE, a dose of 5000 units is given every 8 or 12 hours postoperatively. For the treatment of patients with VTE or ACS, a loading dose of ≈80 units/kg is followed by an infusion of 9 to 18 units/kg/hr, depending on the institution. Begin to monitor aPTT ≈3 to 4 hours after initiation so that any necessary adjustments to the dosage can be made. Generally, treatment is discontinued when the patient is past the risk of thromboembolic complications, but this varies with the specific indication. UFH is most often administered intravenously for 5 to 10 days. If indicated, oral anticoagulation with warfarin is given in conjunction (overlapping) with the heparin infusion because the maximal therapeutic effect of warfarin is not fully reached until after 4 to 5 days (Figure 25-2). Heparin resistance is seen in febrile patients and in those with active thrombosis, phlebitis, infection, MI, cancer, or heparin-induced thrombocytopenia (HIT). Concern has arisen about the possibility of increasing the risk of osteoporosis with prolonged standard heparin administration (>3 months, at an accumulated dose of 20,000 units).
Agents That Act on Blood
Class
Subclass
Generic Name
Trade Name
Heparin group
Heparin
heparin sodium ![]()
generic
Low molecular weight heparin (LMWH)
enoxaparin sodium ![]()
Lovenox
dalteparin
Fragmin
tinzaparin
Innohep
Heparinoids
danaparoid
Orgaran
fondaparinux
AriXtra
Oral anticoagulants
Classic
warfarin ![]()
Coumadin and generics
anisindione
Miradon
Direct thrombin inhibitor (DTI)
dabigatran
Pradaxa ![]()
rivaroxaban
Xarelto
Platelet aggregation inhibitors
Traditional
acetylsalicylic acid (ASA)
aspirin, generic
dipyridamole
Persantine, generic
Extended-release dipyridamole/ASA
Aggrenox
Adenosine diphosphate (ADP)-induced platelet-fibrinogen binding inhibitors
clopidogrel
Plavix ![]()
ticlopidine
Ticlid, generic
prasugrel
Effient, generic
Platelet glycoprotein IIb/IIIa inhibitors (GPIIb/IIIa)
abciximab
ReoPro
eptifibatide
Integrilin
tirofiban
Aggrastat
Phosphodiesterase 3 (PDE3) inhibitors
anagrelide
Agrylin
cilostazol
Pletal
Thrombolytic agents
alteplase (tPA)
Activase
reteplase
Retavase
streptokinase
Streptase
tenecteplase
TNKase
urokinase
Abbokinase
Hemorheologic agent
pentoxifylline
Trental
Therapeutic Overview
Anatomy and Physiology
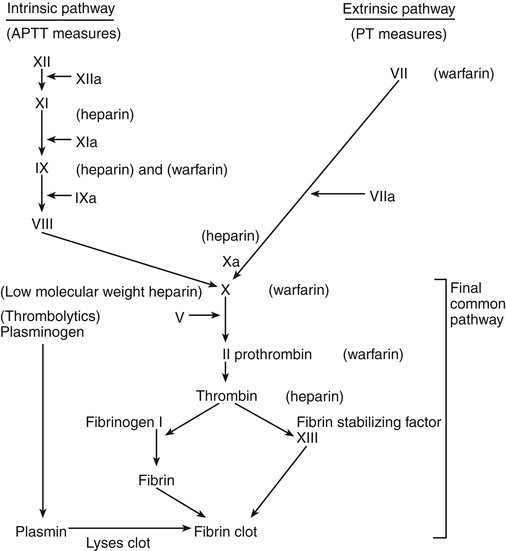
Site of action of antithrombotic and thrombolytic drugs.
Pathophysiology
Assessment
Mechanism of Action
Heparin
Oral Anticoagulants
Factor or Anticoagulant
Half-Life
Factor VII
6 hours
Factor IX
24 hours
Factor X
36 hours
Factor II
50 hours
Protein C
8 hours
Protein S
30 hours
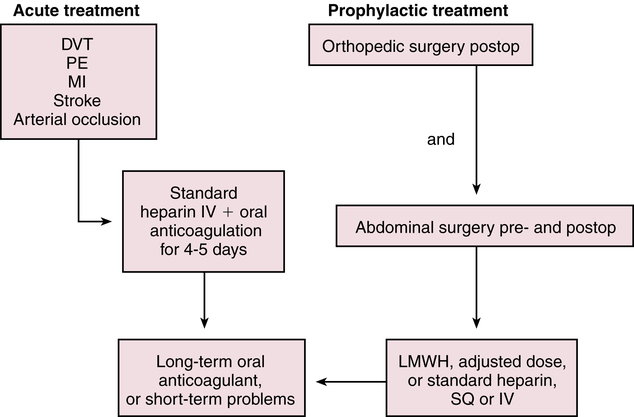
Treatment Principles
Standardized Guidelines
Evidence-Based Recommendations
Cardinal Points of Treatment
Nonpharmacologic and Pharmacologic Treatment
Prevention of VTE
Heparin
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Agents That Act on Blood
Only gold members can continue reading. Log In or Register to continue