Chapter 46 • Stage I: Unilateral involvement • Stage II: Bilateral involvement but no postural abnormalities • Stage III: Bilateral involvement with mild postural imbalance; the patient leads an independent life • Stage IV: Bilateral involvement with postural instability; the patient requires substantial help • Stage V: Severe, fully developed disease; the patient is restricted to bed and chair This tool has largely been replaced by the Unified Parkinson Disease Rating Scale (UPDRS) for following the longitudinal course of PD. This latter tool is much more cumbersome but descriptive. It consists of sections on (1) mentation, behavior, and mood; (2) ADLs; and (3) motor activity. These skills are evaluated by interview. Some sections require assignment of multiple grades to each extremity. A total of 199 points is possible, with 199 representing the worst (total) disability and 0 indicating no disability. Both of these tools may be viewed online through the Massachusetts General Hospital neurosurgical website at http://neurosurgery.mgh.harvard.edu/Functional/pdstages.htm. Selegiline (also called deprenyl), an MAO-B inhibitor, is used with other drugs early in the management of idiopathic PD. The mechanism of action of selegiline is not fully understood. These drugs inhibit the enzyme MAO-B, which breaks down dopamine in the brain. MAO-B inhibitors cause dopamine to accumulate in surviving nerve cells and reduce the symptoms of PD. Studies supported by the National Institute of Neurological Disorders and Stroke (NINDS) have shown that selegiline can delay the need for levodopa therapy by a year or longer. When selegiline or rasagiline is given with levodopa, they appear to enhance and prolong the response to levodopa and thus may reduce wearing off. Another MAO-B inhibitor, rasagiline, was approved by the FDA in May 2006 for use in treating patients with PD (see Table 46-3). Patients with recent, severe brain injuries treated with daily doses of the Parkinson’s disease drug amantadine hydrochloride improved faster than those in the placebo group, according to a report in The New England Journal of Medicine. “The main finding is that on every single behavioral domain measured, we had a higher incidence of recovery in the amantadine group than in the placebo group,” said Joseph Giacino, who led the research team at JFK Johnson Rehabilitation Institute. Rivastigmine is available as an oral or transdermal system (Exelon Patch) for the treatment of patients with mild to moderate dementia associated with PD. See Chapter 42 for complete prescribing information. • Pahwa R et al: Guidelines: practice parameter: Treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology, Neurology 66:983-995, 2006. • Beneficial—immediate-release levodopa • Trade-off between benefits and harms—dopamine agonists, dopamine agonists plus levodopa, MAO-B inhibitors • Entacapone and rasagiline should be offered to reduce off time. • Pergolide, pramipexole, ropinirole, and tolcapone should be considered to reduce off time. • Selegiline, rasagiline, pramipexole, ropinirole, tolcapone, cabergoline, entacapone, and selegiline may be considered to reduce off time and so used when the action of other drugs is wearing off. Apomorphine is used primarily to treat freezing. • Available evidence does not establish the superiority of one medicine over another in reducing off time. • Amantadine may be considered to reduce levodopa-associated dyskinesia (level C). • Deep brain stimulation of the subthalamic nuclei (STN) may be considered to improve motor function and reduce off time, dyskinesia, and medication usage. • Evidence is insufficient to support or refute the efficacy of DBS of the globus pallidus pars interna (GPi) or VIM nucleus of the thalamus in reducing off time, dyskinesia, or medication usage, or in improving motor function. • Preoperative response to levodopa predicts better outcome after DBS of the STN (level B). • Initial drugs: First line—levodopa, dopamine agonists, rasagiline • carbidopa/levodopa: All patients will eventually need; most effective • selegiline: Has a modest effect • rasagiline: Initial monotherapy or as an adjunct to levodopa-carbidopa; to shorten end of dose wearing off • Dopamine agonists: Less likely than levodopa to cause dyskinesias or motor fluctuations • Pramipexole and ropinirole are effective in early and late Parkinson’s. • Apomorphine injection is used for acute treatment of episodes of immobility known as “freezing.” • Anticholinergics: Reduce tremor, rigidity, and drooling • COMT inhibitors: Reduce motor fluctuations in patients with advanced disease but may increase dyskinesias; often used to treat wearing off with Sinemet Management of PD should include programs designed to optimize the patient’s general health, nutrition, and emotional and neuromuscular status. Table 46-1 provides a treatment algorithm. Refer the patient to a neurologist to confirm the diagnosis and to provide guidance with medical management. TABLE 46-1 Basic Treatment Algorithm for Parkinson’s Disease Doses always are titrated slowly according to the patient’s symptoms. Physical therapy can be very beneficial in keeping the patient mobile and keeping weight down. • Age. Patients younger than 65 years of age should be started with a dopamine agonist, whereas treatment for older patients should be initiated with carbidopa/levodopa. Older patients are less likely to develop levodopa-related motor problems. Anticholinergics are contraindicated in older patients because of increased risks of cognitive impairment, blurred vision, and dry mouth. • Cognitive Impairment. Treatment with carbidopa/levodopa should be initiated and polypharmacy eliminated through gradual discontinuation of drugs in the following order: sedative medications, anticholinergics, amantadine, selegiline, dopamine agonists. • Disease Severity. It is acceptable to start with carbidopa/levodopa and to move to a dopamine agonist if needed. • Employment Status. Dopamine agonists could be used first, especially in younger patients who are at greater risk for developing motor complications. Other experts advocate starting with carbidopa/levodopa. • Cost. When financial realities outweigh the potential for future benefits, initiate treatment with the least expensive medications. Cost is a significant variable for patients. Some medications are not covered on insurance formularies; newer medications tend not to be covered by Medicare plans. Some type of prescription assistance program may help some of these patients pay for their prescriptions. • Combined Therapy. When patients treated with single agents (either carbidopa/levodopa or dopamine agonists) require additional treatment, most experts agree that they should receive combination therapy rather than an increased dose of the current drug.
Antiparkinson Agents
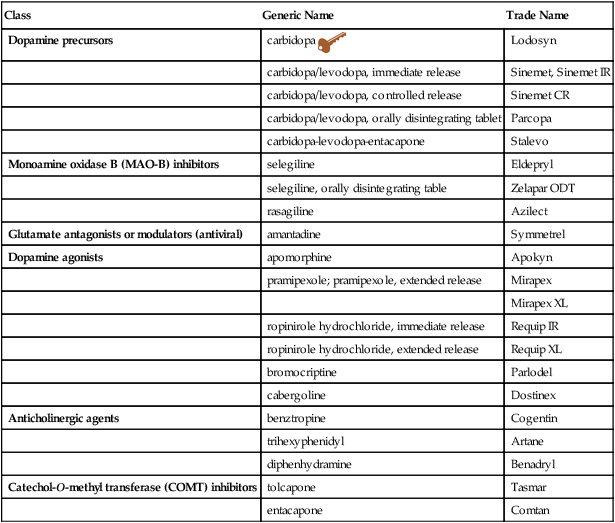
Class
Generic Name
Trade Name
Dopamine precursors
carbidopa ![]()
Lodosyn
carbidopa/levodopa, immediate release
Sinemet, Sinemet IR
carbidopa/levodopa, controlled release
Sinemet CR
carbidopa/levodopa, orally disintegrating tablet
Parcopa
carbidopa-levodopa-entacapone
Stalevo
Monoamine oxidase B (MAO-B) inhibitors
selegiline
Eldepryl
selegiline, orally disintegrating table
Zelapar ODT
rasagiline
Azilect
Glutamate antagonists or modulators (antiviral)
amantadine
Symmetrel
Dopamine agonists
apomorphine
Apokyn
pramipexole; pramipexole, extended release
Mirapex
Mirapex XL
ropinirole hydrochloride, immediate release
Requip IR
ropinirole hydrochloride, extended release
Requip XL
bromocriptine
Parlodel
cabergoline
Dostinex
Anticholinergic agents
benztropine
Cogentin
trihexyphenidyl
Artane
diphenhydramine
Benadryl
Catechol-O-methyl transferase (COMT) inhibitors
tolcapone
Tasmar
entacapone
Comtan
Therapeutic Overview
Anatomy and Physiology
Assessment
Mechanism of Action
Treatment Principles
Standardized Guidelines
Evidence-Based Recommendations
Cardinal Points of Treatment
Nonpharmacologic Treatment
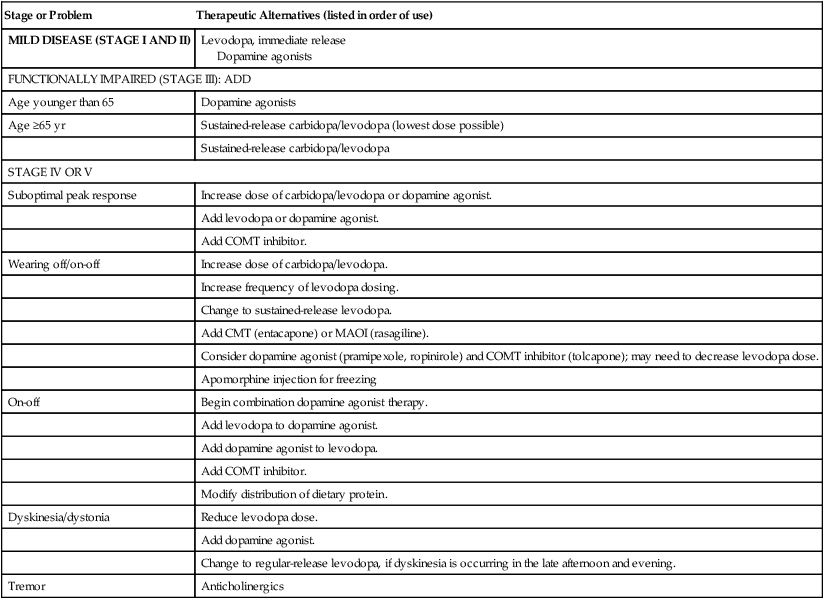
Stage or Problem
Therapeutic Alternatives (listed in order of use)
MILD DISEASE (STAGE I AND II)
Levodopa, immediate release
Dopamine agonists
FUNCTIONALLY IMPAIRED (STAGE III): ADD
Age younger than 65
Dopamine agonists
Age ≥65 yr
Sustained-release carbidopa/levodopa (lowest dose possible)
Sustained-release carbidopa/levodopa
STAGE IV OR V
Suboptimal peak response
Increase dose of carbidopa/levodopa or dopamine agonist.
Add levodopa or dopamine agonist.
Add COMT inhibitor.
Wearing off/on-off
Increase dose of carbidopa/levodopa.
Increase frequency of levodopa dosing.
Change to sustained-release levodopa.
Add CMT (entacapone) or MAOI (rasagiline).
Consider dopamine agonist (pramipexole, ropinirole) and COMT inhibitor (tolcapone); may need to decrease levodopa dose.
Apomorphine injection for freezing
On-off
Begin combination dopamine agonist therapy.
Add levodopa to dopamine agonist.
Add dopamine agonist to levodopa.
Add COMT inhibitor.
Modify distribution of dietary protein.
Dyskinesia/dystonia
Reduce levodopa dose.
Add dopamine agonist.
Change to regular-release levodopa, if dyskinesia is occurring in the late afternoon and evening.
Tremor
Anticholinergics
Pharmacologic Treatment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Antiparkinson Agents
Only gold members can continue reading. Log In or Register to continue
