CHAPTER 40 The Hypothalamus and Pituitary Gland
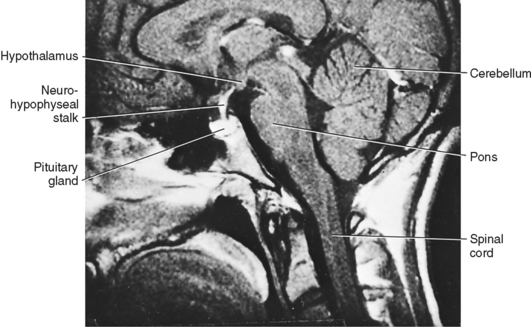
The pituitary gland (also called the hypophysis) is a small (about 0.5 g in weight), yet complex endocrine structure at the base of the forebrain (Fig. 40-1). It is composed of an epithelial component called the adenohypophysis and a neural structure called the neurohypophysis. The adenohypophysis is composed of five cell types that secrete six hormones. The neurohypophysis releases several neurohormones. All endocrine functions of the pituitary gland are regulated by the hypothalamus and by negative- and positive-feedback loops.
ANATOMY
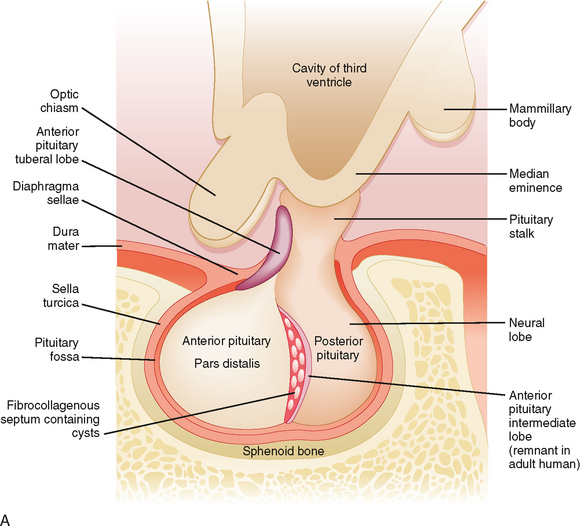
Microscopic examination of the pituitary reveals two distinct types of tissue: epithelial and neural (Fig. 40-2). The epithelial portion of the human pituitary gland is called the adenohypophysis. The adenohypophysis makes up the anterior portion of the pituitary and is often referred to as the anterior lobe of the pituitary, and its hormones are referred to as anterior pituitary hormones. The adenohypophysis is composed of three parts: (1) the pars distalis, which makes up about 90% of the adenohypophysis; (2) the pars tuberalis, which wraps around the stalk; and (3) the pars intermedia, which regresses and is absent in adult humans.
The neural portion of the pituitary is called the neurohypophysis and it represents a down-growth of the hypothalamus. The lowest portion of the neurohypophysis is called the pars nervosa, which is also called the posterior lobe of the pituitary (or simply, the “posterior pituitary”). At the superior end of the neurohypophysis, a funnel-shaped swelling called the median eminence develops. The rest of the neurohypophysis, which extends from the median eminence down to the pars nervosa, is called the infundibulum. The infundibulum and the pars tuberalis make up the pituitary stalk—a physical connection between the hypothalamus and the pituitary gland (Fig. 40-2).
THE NEUROHYPOPHYSIS
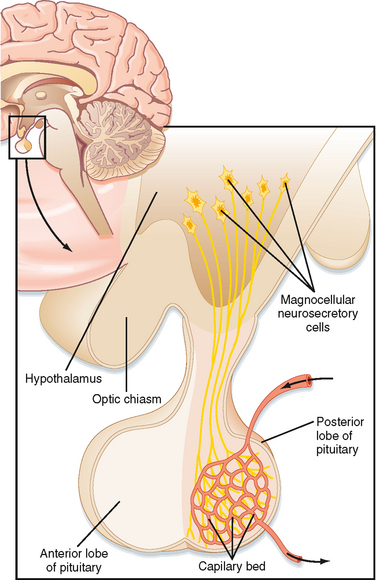
The pars nervosa is a neurovascular structure that is the site of release of neurohormones adjacent to a rich bed of fenestrated capillaries. The peptide hormones that are released are antidiuretic hormone (ADH, or arginine vasopressin) and oxytocin. The cell bodies of the neurons that project to the pars nervosa are located in the supraoptic nuclei (SON) and paraventricular nuclei (PVN) of the hypothalamus (in this context, a “nucleus” refers to a collection of neuronal cell bodies residing within the central nervous system [CNS]—a “ganglion” is a collection of neuronal cell bodies residing outside the CNS). The cell bodies of these neurons are described as magnocellular (i.e., large cell bodies), and they project axons down the infundibular stalk as the hypothalamohypophyseal tracts. These axons terminate in the pars nervosa (Fig. 40-3). In addition to axonal processes and termini from the SON and PVN, there are glial-like supportive cells called pituicytes. The posterior pituitary is extensively vascularized and the capillaries are fenestrated, thereby facilitating diffusion of hormones into the vasculature.
Synthesis of ADH and Oxytocin
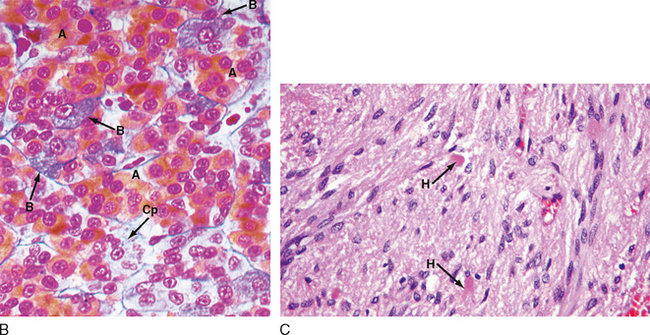
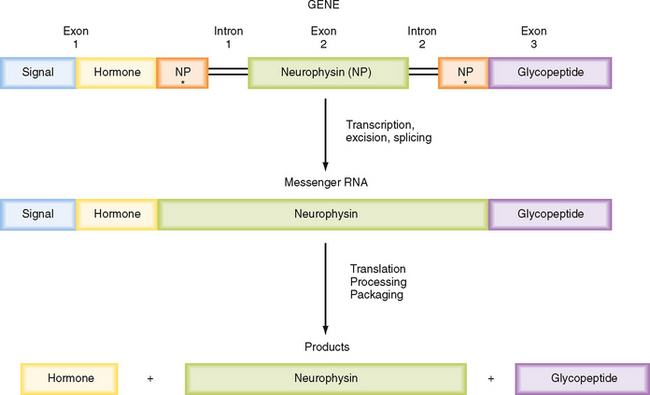
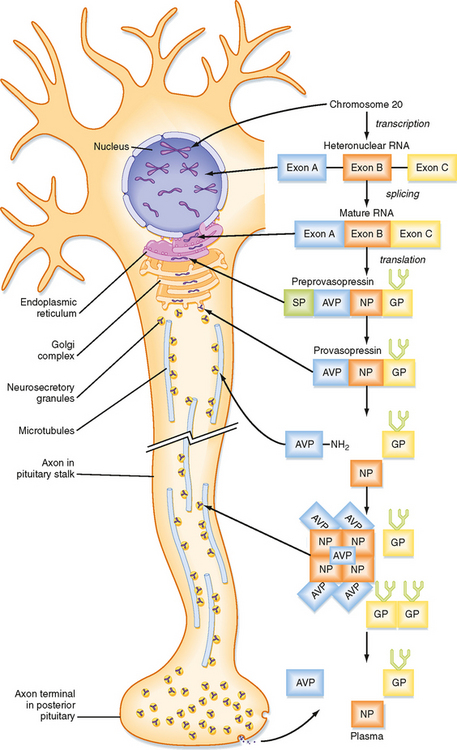
ADH and oxytocin are nonapeptides (nine amino acids) that are similar in structure, differing in only two amino acids. They have limited overlapping activity. ADH and oxytocin are synthesized as preprohormones (Fig. 40-4). Each prohormone harbors the structure of oxytocin or ADH and a cosecreted peptide: either neurophysin I (associated with ADH) or neurophysin II (associated with oxytocin). These preprohormones are called preprovasophysin and preprooxyphysin. The N-signal peptide is cleaved as the peptide is transported into the endoplasmic reticulum. The prohormone is packaged in the endoplasmic reticulum and Golgi apparatus in a membrane-bound secretory granule in cell bodies within the SON and PVN (Fig. 40-5). The secretory granules are conveyed intraaxonally through a “fast” (i.e., millimeters per hour) ATP-dependent transport mechanism down the infundibular stalk to axonal termini in the pars nervosa. During transit of the secretory granule, the prohormones are proteolytically cleaved to produce equimolar amounts of hormone and neurophysin. Secretory granules containing fully processed peptides are stored in the axonal termini. Axonal swelling because of the storage of secretory granule can be observed by light microscopy and is termed Herring bodies.
ADH and oxytocin are released from the pars nervosa in response to stimuli that are primarily detected at the cell body and its dendrites in the SON and PVN of the hypothalamus. The stimuli are mainly in the form of neurotransmitters released from hypothalamic interneurons. With sufficient stimulus, the neurons will depolarize and propagate an action potential down the axon. At the axonal termini, the action potential increases intracellular [Ca++] and results in a stimulus-secretion response, with the exocytosis of ADH or oxytocin, along with neurophysins, into the extracellular fluid of the pars nervosa (Fig. 40-5). Hormones and neurophysins enter the peripheral circulation, and both can be measured in blood.
Actions and Regulation of ADH and Oxytocin
ADH primarily acts at the kidney to retain water (antidiuresis). The actions of ADH and regulation of ADH secretion were described in Chapter 34. Oxytocin primarily acts on the pregnant uterus (labor inducing) and myoepithelial cells of the breast (milk let-down during nursing). The actions and regulation of oxytocin are discussed in Chapter 43.
THE ADENOHYPOPHYSIS
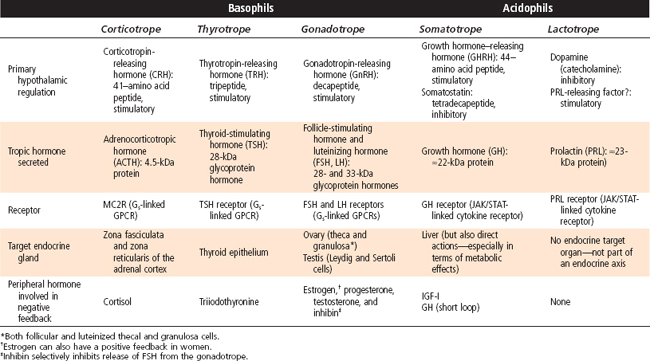
The pars distalis is composed of five endocrine cell types that produce six hormones (Table 40-1). Because of the histological characteristics of the cell types, the corticotropes, thyrotropes, and gonadotropes are referred to as pituitary basophils, whereas the somatotropes and lactotropes are referred to as pituitary acidophils (Fig. 40-2, B).
Table 40-1 Cell Types of the Adenohypophysis: Hormonal Production and Action, Hypothalamic Regulation, and Feedback Regulation
Endocrine Axes
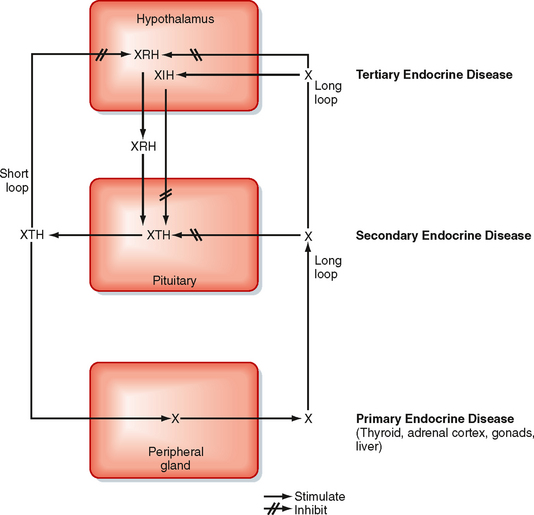
Before discussing the individual hormones of the adenohypophysis, it is important to understand the structural and functional organization of the adenohypophysis within the endocrine axes (discussed briefly in Chapter 37; also refer to Table 40-1 and Fig. 40-6). Each endocrine axis is composed of three levels of endocrine cells: (1) hypothalamic neurons, (2) anterior pituitary cells, and (3) peripheral endocrine glands. Hypothalamic neurons release specific hypothalamic releasing hormones (XRHs) that stimulate the secretion of specific pituitary tropic hormones (XTHs). In some cases, production of a pituitary tropic hormone is secondarily regulated by a release-inhibiting hormone (XIH). Pituitary tropic hormones then act on specific peripheral target endocrine glands and stimulate them to release peripheral hormones (X). The peripheral hormone X has two general functions: it regulates several aspects of human physiology, and it negatively feeds back on the pituitary gland and hypothalamus to inhibit the production and secretion of tropic hormones and releasing hormones, respectively (Fig. 40-6).
Significant progress has been made in understanding the differentiation of the five endocrine cells of the pars distalis from one precursor cell. The homeodomain transcription factor Prop-1 is expressed soon after Rathke’s pouch forms and promotes the cell lineages of somatotropes, lactotropes, thyrotropes, and gonadotropes. In humans, rare mutations in the Prop-1 gene result in a type of combined pituitary hormone deficiency. These individuals display dwarfism because of lack of GH, mental retardation secondary to hypothyroidism, and infertility as a result of a lack of gonadotropins. A subsequently expressed, pituitary-specific gene product called Pit-1 was identified in mice. Pit-1 and its human homologue POUF1 are also homeodomain transcription factors. POUF1 is absolutely required for the differentiation of thyrotropes, somatotropes, and lactotropes, and it directly stimulates the transcription and expression of TSH, GH, and prolactin. Affected individuals with POUF1 mutations have dwarfism and mental retardation. The orphan nuclear hormone receptor-related transcription factor steroidogenic factor-1 (SF-1) was originally identified in the adrenal cortex and gonads as a regulator of steroidogenic enzyme gene expression. However, SF-1 is also expressed in GnRH neurons in the hypothalamus and pituitary gonadotropes. SF-1 regulates the transcription of LH and FSH. Mutations in the SF-1 gene disrupt adrenal and gonadal function, including the loss of gonadotropes in the pituitary gland. Tpit is a transcription factor involved in the differentiation of corticotropes. Tpit interacts with other transcription factors to promote the differentiation of corticotropes and expression of the POMC gene (see later). Mutations in the human Tpit gene result in isolated ACTH deficiency (i.e., other cell types in the body that also express the POMC gene are not affected). This results in a form of secondary adrenal insufficiency that requires life-long replacement with glucocorticoids (see Chapter 42).
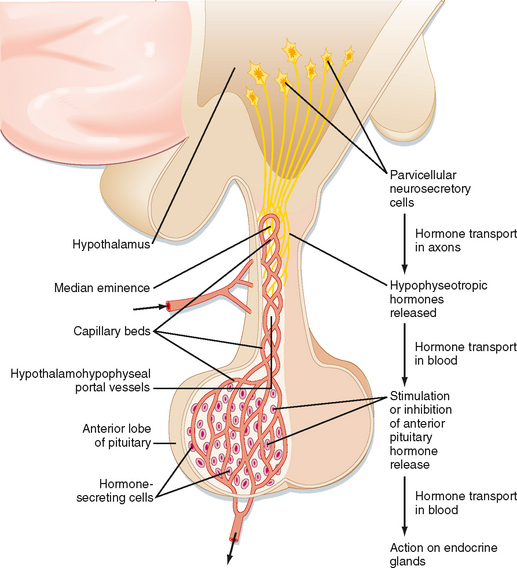
The hypothalamic level of regulation is neurohormonal. Collections of neuronal cell bodies (called nuclei) reside in several regions of the hypothalamus and are collectively referred to as the hypophysiotropic (i.e., “stimulatory to the hypophysis” [= pituitary]) region of the hypothalamus. These nuclei are distinguished from the magnocellular neurons of the PVN and SON that project to the pars nervosa in that they have small, or parvicellular, neuronal cell bodies that project axons to the median eminence. Parvicellular neurons secrete releasing hormones from their axonal termini at the median eminence (Fig. 40-7). The releasing hormones enter a primary plexus of fenestrated capillaries and are then conveyed to a second capillary plexus located in the pars distalis by the hypothalamohypophyseal portal vessels (a “portal” vessel is defined as a vessel that begins and ends in capillaries without going through the heart). At the secondary capillary plexus, the releasing hormones diffuse out of the vasculature and bind to their specific receptors on specific cell types within the pars distalis. The neurovascular link (i.e., the pituitary stalk) between the hypothalamus and pituitary is somewhat fragile and can be disrupted by physical trauma, surgery, or hypothalamic disease. Damage to the stalk and subsequent functional isolation of the anterior pituitary result in a decline in all anterior pituitary tropic hormones except prolactin (see later).
The cells of the adenohypophysis make up the intermediate level of an endocrine axis. The adenohypophysis secretes protein hormones that are referred to as tropic hormones—ACTH, TSH, FSH, LH, GH, and PRL (Table 40-1). With a few exceptions, tropic hormones bind to their cognate receptors on peripheral endocrine glands. Because of this arrangement, pituitary tropic hormones generally do not directly regulate physiological responses (see Chapter 37).
The endocrine axes have the following important features:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree