CHAPTER 28 The Gastric Phase of the Integrated Response to a Meal
In this chapter we will study gastrointestinal (GI) tract physiology when food is in the stomach (i.e., the gastric phase of digestion). This chapter discusses gastric function and its regulation, in addition to changes in function that occur in more distal regions of the GI tract. The main functions of the stomach are to act as a temporary reservoir for the meal and to initiate protein digestion through the secretion of acid and the enzyme precursor pepsinogen. Other functions are listed in Table 28-1.
Table 28-1 Functions of the Stomach
FUNCTIONAL ANATOMY OF THE STOMACH
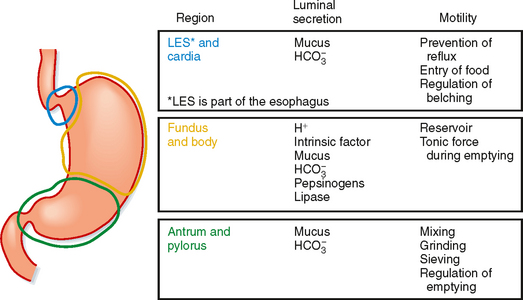
The stomach is divided into three regions: the cardia, the corpus (also referred to as the fundus or body), and the antrum (Fig. 28-1). However, when discussing the physiology of the stomach, it is helpful to think of it as subdivided into two functional regions: the proximal and distal parts of the stomach. The proximal portion of the stomach (called proximal because it is the most cranial) and the distal portion of the stomach (furthest away from the mouth) have quite different functions in the postprandial response to a meal, which will be discussed later.
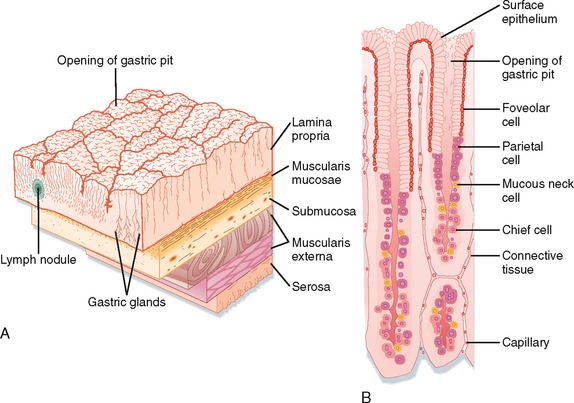
The lining of the stomach is covered with a columnar epithelium folded into gastric pits; each pit is the opening of a duct into which one or more gastric glands empty (Fig. 28-2). The gastric pits account for a significant fraction of the total surface area of the gastric mucosa. The gastric mucosa is divided into three distinct regions based on the structure of the glands. The small cardiac glandular region, located just below the lower esophageal sphincter (LES), primarily contains mucus-secreting gland cells. The remainder of the gastric mucosa is divided into the oxyntic or parietal (acid-secreting) gland region, located above the gastric notch (equivalent to the proximal part of the stomach), and the pyloric gland region, located below the notch (equivalent to the distal part of the stomach).
The structure of a gastric gland from the oxyntic glandular region is illustrated in Figure 28-2. Surface epithelial cells extend slightly into the duct opening. The opening of the gland is called the isthmus and is lined with surface mucous cells and a few parietal cells. Mucous neck cells are located in the narrow neck of the gland. Parietal or oxyntic cells, which secrete HCl and intrinsic factor (involved in absorption of vitamin B12), and chief or peptic cells, which secrete pepsinogens, are located deeper in the gland. Oxyntic glands also contain enterochromaffin-like (ECL) cells, which secrete histamine, and D cells, which secrete somatostatin. Parietal cells are particularly numerous in glands in the fundus, whereas mucus-secreting cells are more numerous in glands of the pyloric (antral) glandular region. In addition, the pyloric glands contain G cells, which secrete the hormone gastrin. The parietal glands can also be divided into regions: the neck (mucous neck cells and parietal cells) and the base (peptic/chief and parietal cells). Endocrine cells are scattered throughout the glands.
GASTRIC SECRETION
The fluid secreted into the stomach is called gastric juice. Gastric juice is a mixture of the secretions of the surface epithelial cells and the secretions of gastric glands. One of the most important components of gastric juice is H+, a secretion that occurs in the face of a very large concentration gradient. Thus, H+ secretion by the parietal mucosa is an energy-intensive process. The cytoplasm of the parietal cell is densely packed with mitochondria, which have been estimated to fill 30% to 40% of the cell’s volume. One major function of H+ is conversion of inactive pepsinogen (the major enzyme product of the stomach) to pepsins, which initiate protein digestion in the stomach. Additionally, ions are important for preventing invasion and colonization of the gut by bacteria and other pathogens that may be ingested with food. The stomach also secretes significant amounts of HCO3− and mucus, important for protection of the gastric mucosa against the acidic and peptic luminal environment. However, in a healthy human the only gastric secretion required is intrinsic factor, which is necessary for the absorption of vitamin B12 (cobalamin). The functions of other components of gastric juice are redundant with secretions provided more distally in the GI tract.
Composition of Gastric Secretions
Organic Constituents of Gastric Juice
The predominant organic constituent of gastric juice is pepsinogen, the inactive proenzyme of pepsin. Pepsins, often collectively called “pepsin,” are a group of proteases secreted by the chief cells of the gastric glands. Pepsinogens are contained in membrane-bound zymogen granules in the chief cells. Zymogen granules release their contents by exocytosis when chief cells are stimulated to secrete (Table 28-2). Pepsinogens are converted to active pepsins by the cleavage of acid-labile linkages. The lower the pH, the more rapid the conversion. Pepsins also act proteolytically on pepsinogens to form more pepsin. Pepsins are most proteolytically active at pH 3 and below. Pepsins may digest as much as 20% of the protein in a typical meal but are not required for digestion because their function can be replaced by that of pancreatic proteases. When the pH of the duodenal lumen is neutralized, pepsins are inactivated by the neutral pH.
Table 28-2 Stimulation of Chief Cells in the Integrated Response to a Meal
| Stimulant | Source |
|---|---|
| Acetylcholine (ACh) | Enteric neurons |
| Gastrin | G cells in the gastric antrum |
| Histamine | ECL cells in the gastric corpus |
| Cholecystokinin (CCK) | I cells in the duodenum |
| Secretin | S cells in the duodenum |
Cellular Mechanisms of Gastric Acid Secretion
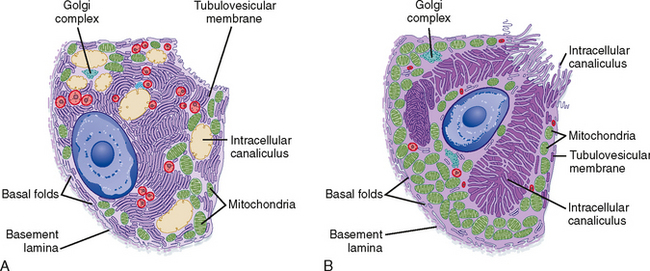
Parietal cells have a distinctive ultrastructure (Fig. 28-3). Branching secretory canaliculi course through the cytoplasm and are connected by a common outlet to the cell’s luminal surface. Microvilli line the surfaces of the secretory canaliculi. The cytoplasm of unstimulated parietal cells contains numerous tubules and vesicles, which is called the tubulovesicular system. The membranes of tubulovesicles contain the transport proteins responsible for secretion of H+ and Cl− into the lumen of the gland. When parietal cells are stimulated to secrete HCl (Fig. 28-3), tubulovesicular membranes fuse with the plasma membrane of the secretory canaliculi. This extensive membrane fusion greatly increases the number of H+-K+ antiporters in the plasma membrane of the secretory canaliculi. When parietal cells secrete gastric acid at the maximal rate, H+ is pumped against a concentration gradient that is about 1 million—fold. Thus, the pH is 7 in the parietal cell cytosol and 1 in the lumen of the gastric gland.
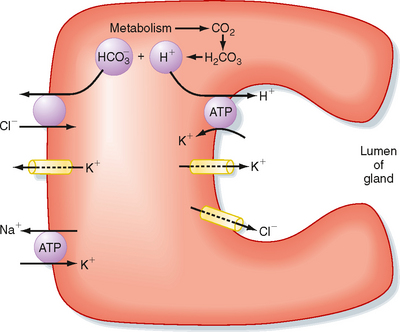
The cellular mechanism of H+ secretion by the parietal cell is depicted in Figure 28-4. Cl− enters the cell across the basolateral membrane in exchange for HCO3− generated in the cell by the action of carbonic anhydrase, which produces HCO3− and H+. H+ is secreted across the luminal membrane by H+,K+-ATPase in exchange for K+. Cl− enters the lumen via an ion channel (a ClC Cl− channel) located in the luminal membrane. Increased intracellular Ca++ and cAMP stimulate luminal membrane conduction of Cl− and K+. Increased K+ conductance hyperpolarizes the luminal membrane potential, which increases the driving force for efflux of Cl− across the luminal membrane. The K+ channel in the basolateral membrane also mediates the efflux of K+ that accumulates in the parietal cell via the activity of H+,K+-ATPase. In addition, cAMP and Ca++ promote the trafficking of Cl− channels into the luminal membrane and the fusion of cytosolic tubulovesicles containing H+,K+-ATPase with the membrane of the secretory canaliculi (Figs. 28-3 and 28-4). Parietal cell secretion of H+is also accompanied by transport of HCO3− into the bloodstream to maintain intracellular pH.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree