The Eye
J. Douglas Cameron, M.D., MBA
Raymond G. Areaux Jr., M.D.
INTRODUCTION
The observations and opinions of surgical pathologists are critical in managing many pediatric ocular conditions including potentially fatal entities such as retinoblastoma and suspected nonaccidental trauma.
Because pediatric ophthalmic surgical specimens tend to be infrequent, this chapter includes a discussion of pertinent ocular anatomy and pivotal events in embryologic development of the eye; the intention is to provide a context for pathologic features. In addition, the type of surgical procedure used to obtain the tissue specimen is described to assist in understanding the origin of the specimen and orientation of gross specimens.
This chapter is organized by the types of tissue most frequently received in the laboratory: eyelid tissue, conjunctiva, cornea, vitreous, orbital soft tissues, whole globes removed surgically, and globes removed at autopsy. Crystalline lens tissue removed because of congenital cataracts and extraocular muscle tissues removed during some types of strabismus procedures are infrequently processed because histologic observations of this type of specimen are not relevant to management of the ocular abnormality.
THE EYELID
Structure of the Eyelid
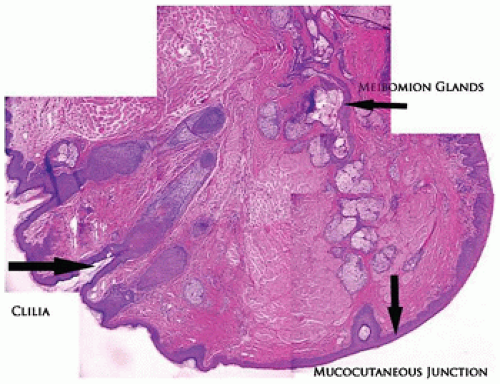
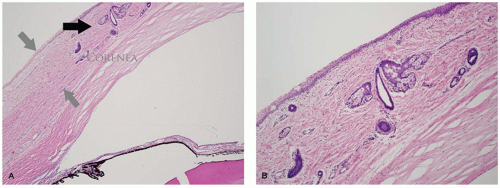
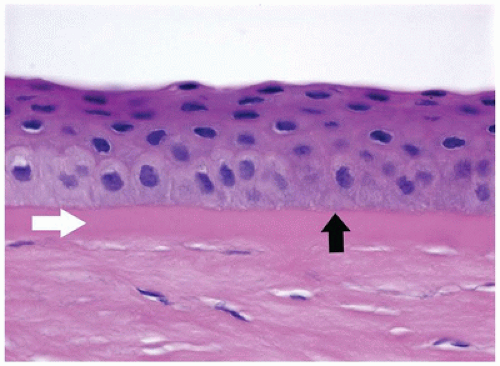
The eyelid is covered by stratified squamous epithelium associated with a thin keratin layer. The surface merges with the mucous membrane at the mucocutaneous junction located on the eyelid margin (Figure 11-1). The epidermis is associated with pilosebaceous units, eccrine glands, and apocrine glands. The apocrine glands have no recognized function in the eyelid tissues. The cilia are the product of a modified pilosebaceous unit in that there are no associated piloerector muscles and there is a prominent sebaceous component (glands of Zeis). The tarsal plate is a dense collagenous structure that supports the delicate eyelid and houses a large volume of sebaceous glands (the meibomian glands). No cartilage is present in the eyelid. The holocrine secretion of the meibomian gland is applied to the tear film surface from pores located along the eyelid margin anterior to the mucocutaneous junction and posterior to the eyelid cilia (1,2).
Vascular Abnormalities of the Eyelid
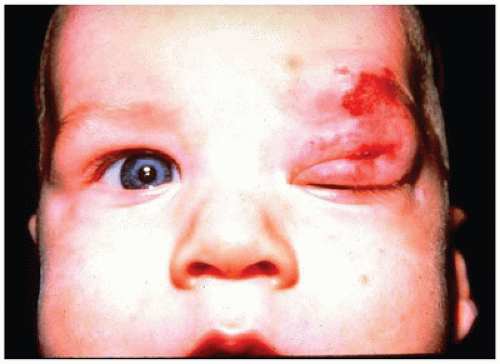
Infantile hemangioma is a benign proliferation of blood vessels of the soft tissue of the face that may involve both eyelids and the orbit. The proliferation does not usually involve the contents of the globe. The cutaneous lesions are red and lobulated and may markedly distort the contours of the face (Figure 11-2). The lesions are rarely biopsied for risk of hemorrhage but occasionally may be surgically debulked. Initially, there is capillary lesion in a lobular pattern characterized by proliferation of endothelial cells around a small-caliber vascular channel. The lesion is not encapsulated, and lobules of the hemangioma extend into the surrounding soft tissue. With time, the endothelial profile flattens and the lumen becomes more prominent as interstitial tissue develops. The lesions appear clinically within the first month of life and may enlarge over several years before spontaneously involuting by approximately age 7 years. In the interim, obstruction of vision by mechanical eyelid ptosis or secondary astigmatism from mechanical distortion of the cornea may interfere with normal visual development resulting in amblyopia (developmental arrest of visual function, which may become permanent without treatment, “lazy eye”) (3).
Surgical treatment of these lesions in children requires extreme precision. Steroids injected into the lesion may be seen as amorphous material in the vascular lumen or in the interstitial space (4). Central retinal artery occlusion has been reported with intralesional injections (5). Since 2008, beta-blockers have become the most common treatment for inducing regression of hemangiomas (6). Superficial lesions may be treated topically without surgical intervention (7).
Nevus flammeus is a congenital vascular lesion in the distribution of the first and second divisions of the trigeminal nerve. The vascular abnormality is present at birth and does not progress or regress. The cutaneous lesion may be treated with laser but is generally not biopsied (8). Nevus flammeus is clinically important because this vascular malformation
is frequently associated with ipsilateral glaucoma and ipsilateral choroidal hemangioma. Glaucoma results from ipsilateral elevation of episcleral venous pressure. The choroidal component is difficult to treat and may progress to serious retinal detachment, a potential cause of loss of vision (9).
is frequently associated with ipsilateral glaucoma and ipsilateral choroidal hemangioma. Glaucoma results from ipsilateral elevation of episcleral venous pressure. The choroidal component is difficult to treat and may progress to serious retinal detachment, a potential cause of loss of vision (9).
Inflammatory Abnormalities of the Eyelid
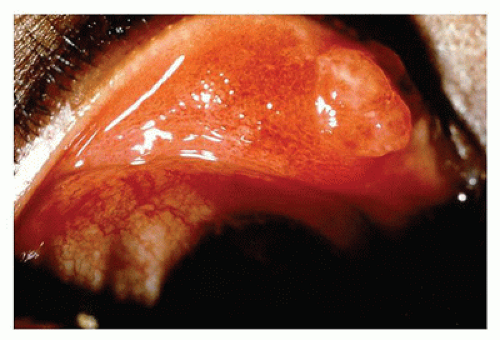
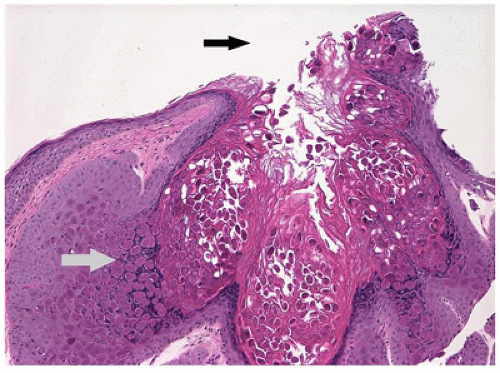
Pyogenic granuloma refers to a polypoid lobular capillary hemangioma of the skin in the discipline of dermatopathology. This term is used by clinical ophthalmologists to describe a granulation tissue reaction usually located in the tarsal conjunctiva, which is a response to mechanical trauma or to the presence of a chalazion (Figure 11-3). Pyogenic granuloma is an impaired wound healing process (10). A reddish lobulated mass develops on the conjunctival surface that may be large enough to protrude through the interpalpebral fissure. The overlying mucous membrane may show effects of drying and reactive squamous proliferation. In the subepithelial tissue, there are acute and chronic inflammatory reactions associated with multiple delicate vascular channels and an edematous stroma (granulation tissue). Conjunctival pyogenic granuloma will usually spontaneously involute over days to weeks. Topical steroids may be prescribed in the acute inflammatory phase to encourage involution. The area may be excised later if the mass interferes with surface lubrication of the eye or results in malpositioning of the eyelid margin or unacceptable appearance.
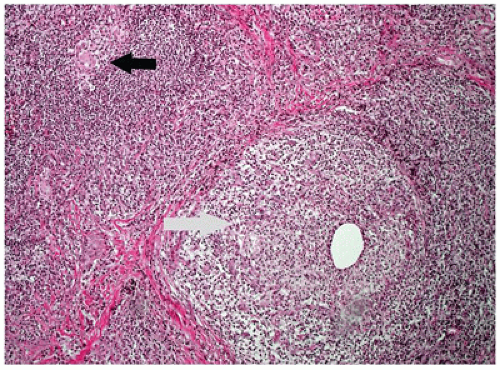
Chalazion is a granulomatous reaction to sebaceous products of the meibomian gland of the eyelid. This condition is often misinterpreted as a “sty,” which is an abscess of a pilosebaceous unit. The meibomian gland duct becomes occluded, and sebaceous gland content ruptures into the surrounding soft tissue. The affected area is initially tender but evolves into a firm nontender nodule. The tissue is usually friable amorphous gray tissue composed of a lipogranulomatous reaction with foreign body and occasional Langhans-type giant cells (Figure 11-4). The surrounding normal tissue is usually not represented in the specimen; however, infiltration among the fibers of the orbicularis muscle may be observed occasionally.
Generally, the nodule resolves over weeks or months and responds to warm compresses and scrubbing of the eyelid margin. In some individuals, there may be multiple episodes in different locations in the eyelid. In adults, multiple chalazia occurring in the same location is a risk factor for sebaceous carcinoma. In children, chalazia may mechanically induce refractive error or ptosis or contribute to progressive ptosis. Thus, incision and curettage may be performed if there is risk of amblyopia (11).
Juvenile xanthogranuloma (nevoxanthoendothelioma) is a non-Langerhans cell histiocytosis (LCH) but shares a common CD34 precursor cell with LCH and may occur in adults as well (12). Well-defined purple to red nodules appear on the skin and anterior surface of the eye (13). The reaction may also appear in the orbit and in the uveal tract. Lesions in the iris may cause spontaneous hyphema, which may be bilateral. The cutaneous lesions are characterized by mononuclear lipidized or nonlipidized cells and Touton giant cells. The lesions tend to involute spontaneously and may respond to topical or periocular steroids. Occasionally, intraocular lesions cause intractable glaucoma requiring enucleation. The mononuclear cells are usually limited to the uveal tract but may involve adjacent structures as well (see chapter 25).
Molluscum contagiosum is a poxvirus infection of the epithelium of the skin in children aged 1 to 4 years with risk factors of swimming and eczema (14). Multiple, well-demarcated, elevated, umbilicated, nontender nodules develop on the skin. Shedding of viral particles from infected epithelial cells near the lid margin may produce a localized, persistent, follicular conjunctivitis, which brings the patient to clinical attention. This infection is often associated with immune deficiency. The lesion consists of acanthotic stratified squamous epithelial cells with prominent intracytoplasmic inclusions (Figure 11-5). The contents of infected cells desquamate into the environment from the umbilicated region. Conjunctival follicular reaction from molluscum contagiosum is difficult to treat medically. Lid margin lesions often require surgical excision.
A sty or hordeolum is an abscess of one of the adnexal units of the eyelid skin. This condition is very infrequent and is generally not biopsied. An external hordeolum is superficial, and an internal hordeolum is located deeper in the eyelid skin. In most cases, a lesion described clinically as a sty may actually be a chalazion (see above).
The orbital septum is a fibrous diaphragm extending from the periosteum of the orbital rim to the eyelid margin. Its major function is to compartmentalize and protect orbital fat from external influences. Preseptal cellulitis is a bacterial infection of the subcutaneous tissue of the eyelid anterior to the orbital septum that may present with a dramatic increase in preseptal and facial soft tissue volume and even abscess. In contradistinction, infection posterior to the orbital septum (orbital cellulitis) causes minimal facial swelling but marked swelling of the intraorbital tissues forcing the globe to move anteriorly (proptosis or exophthalmos). The malposition of the globe as well as direct inflammation of extraocular muscles may limit ocular motility (ophthalmoparesis) resulting in double vision (diplopia). Orbital cellulitis most commonly results from secondary spread of sinusitis across the lamina papyracea of the medial orbital wall, bacterial seeding from trauma that violates the orbital septum or orbital walls, or more rarely from hematogenous spread (15). The orbital septum is a profound barrier to inflammation; it is extremely rare for a preseptal cellulitis to seed the postseptal space without a traumatic bridge in an immunocompetent host. The infected tissue is rarely biopsied, although fine needle aspirations may be used for culturing microorganisms. The most common organisms found are Haemophilus influenzae and Streptococcus species. Treatment is with intravenous systemic antibiotics and close inpatient monitoring. Functional compromise of the globe (e.g., worsening vision, an afferent pupillary defect, markedly elevated intraocular pressure) is evidence of a sight-threatening compartment syndrome that requires urgent surgical decompression.
Neoplastic Lesions of the Eyelid
Melanocytic nevus is a proliferation of abnormal melanocytes at the dermal-epidermal junction. Clinically, the lesions appear as hyperpigmented areas of the skin that
vary in degree and extent of pigmentation (see Chapter 26). Children are infrequently affected; however, the distinction from melanoma by histologic criteria is difficult (16).
vary in degree and extent of pigmentation (see Chapter 26). Children are infrequently affected; however, the distinction from melanoma by histologic criteria is difficult (16).
Spindle cell and epithelioid nevus (Spitz nevus) is a proliferation of melanocytes that has many histologic features of melanoma even though the clinical course is usually benign. The lesion may present as a rapidly enlarging, well-demarcated nodule of the eyelid skin (17). Histologic patterns include spindle and epithelioid cell as well as mixed types. The mitotic rate is usually low, and actively dividing cells are generally located near the superficial dermis. The size of the melanocytes usually diminishes toward the base of the lesion. Regional lymph node metastasis has been reported; however, even in those cases, the long-term course remains favorable. Cutaneous malignant melanoma of the skin may arise in childhood (18) (see Chapter 26).
Oculodermal melanocytosis is congenital hyperpigmentation of the skin associated with involvement of the ipsilateral episcleral tissue located deep to the conjunctiva. Hyperpigmentation is present at birth and generally remains stationary in intensity and extent throughout the life of the individual. The lesions may become more prominent during puberty (19). An increased concentration of typical melanocytes is located at the dermal-epidermal junction and in the episcleral tissue. There is minimal risk of malignant transformation of cutaneous and episcleral melanocytes. There is an increased risk for ipsilateral uveal melanoma, particularly for Caucasians. Malignant transformation may also occur in the nevus itself as well as orbit, optic nerve, and brain (20).
Xeroderma pigmentosum is an autosomal recessive defect in the DNA repair systems of the body. Exposure to ultraviolet light induces the formation of basal cell carcinoma, squamous cell carcinoma, and malignant melanoma in the facial skin of even young children (21). The number of lesions is characteristically large. Children may also develop squamous cell carcinoma of the conjunctiva as well as pterygia. Ocular surface scarring from these lesions may significantly affect visual function (22). There is no treatment to replace the deficient DNAse that normally repairs ultraviolet-damaged DNA. Treatment of individual lesions is accomplished by standard surgical therapy. Prevention is attempted by limiting exposure of the facial skin to sunlight.
Basal cell nevus syndrome (Gorlin-Goltz) is an autosomal dominant condition associated with the development of basal cell carcinoma at multiple sites (22). Basal cell carcinoma of the eyelid has been observed in a 16-year-old. The eyelid lesions tend to be aggressive and may involve the orbit (23). In addition to cutaneous malignancies, there are associated skeletal abnormalities such as odontogenic cysts of the jaw and bifid ribs. Palmar and plantar pits as well as cognitive impairment and intracranial calcifications may be present. As with other ectodermal dysplasia syndromes, the ocular surface may be abnormal due to ocular surface abnormalities. Degenerative pannus (scarring between corneal epithelium and Bowman membrane) may be associated with loss of vision.
Neurofibromatosis type I (NF-1) is an autosomal dominant condition in which various types of abnormally produced cytokines lead to the development of neoplasia of various types. The syndrome is recognized by the presence of hyperpigmented cutaneous regions with a smooth contour (café au lait) spots and proliferation of elements of peripheral nerve (neurofibroma) within the eyelid skin. The neurofibromas are acquired and consist of nodular or plexiform patterns. The nodular form generally does not affect eyelid function. The plexiform variety may produce massive enlargement of the soft tissues of the face including the eyelid, causing major deformations of the eyelid margin (ptosis, ectropion, or entropion), which can result in amblyopia via visual axis occlusion, induction of astigmatism, or corneal scarring. Surgical debulking of lesions is occasionally performed to improve eyelid function, which is often only partially successful. The lesion consists of proliferation of all cellular elements of the peripheral nerve including axons, Schwann cells, and perineural cells. Occasionally, the native peripheral nerve trunk can be identified. The lesions of plexiform deformity are often progressive. In this tissue as well as elsewhere, there is a risk of developing malignant peripheral nerve sheath tumors (see chapter 25).
Optic pathway glioma may be found in 15% to 20% of individuals with NF-1 and may account for significant morbidity in young children. Symptoms include vision loss, proptosis, and precocious puberty (24). Proptosis and glaucoma have been reported on the ipsilateral side of orbitofacial NF-1 (25). The association of NF-1 and uveal melanoma appears to be coincidental (26) (see Chapter 10).
Surgical Procedures of the Eyelid
An eyelid biopsy may be a simple removal of an ellipse of skin because of the suspicion of cutaneous neoplasm. These specimens are handled as are cutaneous biopsies elsewhere. When a lesion involves the eyelid margin, particularly near the punctum, the surgery becomes more complicated because scarring in the region of a punctum may cause lacrimal drainage abnormalities (chronic tearing, epiphora) and because scarring of the eyelid margin may damage the cornea. Some type of superficial lamellar dissection in the region of the punctum may be done to spare punctal function. For lid margin lesions, a full-thickness wedge of eyelid is removed because restoration of lid margin function is facilitated. A lid-splitting procedure removes the tissue either anterior or posterior to the anterior border of the tarsal plate. Full-thickness and partial-thickness lid margin specimens are generally oriented perpendicular to the lid margin (or row of cilia) with nasal and lateral surgical margins. During pediatric ptosis repair surgery, the levator palpebrae superioris and underlying Muller’s muscles may be partially resected to elevate the eyelid. Submitted specimens from patients with congenital ptosis are remarkable for fibro-fatty infiltration of the levator muscle tissue.
THE EYE
Structure of the Eye
The eye (globus oculi) is an extension of the brain that collects and transmits images gathered from the environment.
Two elements are essential: a method of focusing light (the anterior segment) and method of detecting and transferring images from the external environment to the brain (posterior segment). The anterior segment is composed of the cornea, the anterior chamber, the iris, the posterior chamber, and the crystalline lens (Figure 11-6). These structures transmit and refract light by reorienting parallel rays of light to a focal point. The posterior segment is composed of the vitreous, the retina, the optic disc, the uveal tract, and the sclera. These structures convert energy from a restricted portion of the electromagnetic spectrum via a photochemical process into impulses that can be integrated with the electrochemical processes of the brain (1,2).
Two elements are essential: a method of focusing light (the anterior segment) and method of detecting and transferring images from the external environment to the brain (posterior segment). The anterior segment is composed of the cornea, the anterior chamber, the iris, the posterior chamber, and the crystalline lens (Figure 11-6). These structures transmit and refract light by reorienting parallel rays of light to a focal point. The posterior segment is composed of the vitreous, the retina, the optic disc, the uveal tract, and the sclera. These structures convert energy from a restricted portion of the electromagnetic spectrum via a photochemical process into impulses that can be integrated with the electrochemical processes of the brain (1,2).
THE CONJUNCTIVA
Structure of the Conjunctiva
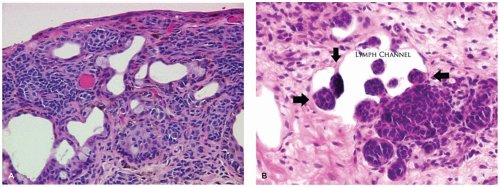
The conjunctiva extends from the eyelid margin to the junction of the cornea and sclera (the limbus). The conjunctiva is a mucous membrane with many specialized regions. Along the internal lining of the eyelid (the tarsal conjunctiva), the surface is tightly adherent to the tarsal plate. There is redundant conjunctiva at the forniceal regions of the eyelid to allow full mobility of the globe. The conjunctiva over the globe (bulbar conjunctiva) is loosely applied. The associated accessory lacrimal tissue is regional and clinically inconspicuous. There is associated nonnodal lymphoid tissue in the subepithelial space, particularly in the region of the fornix. The conjunctival surface is composed of stratified, nonkeratinizing epithelium containing a variable number of intraepithelial goblet cells found most prominently in the bulbar conjunctiva (Figure 11-7). Dendritic melanocytes and antigen-processing cells (Langerhans cells) are present throughout the surface epithelium. The underlying tissue is nonspecific, delicate fibrovascular tissue (substantia propria). The substantia propria of the conjunctiva fuses with the fibrovascular tissue of the globe (episcleral tissue and Tenon capsule) at the limbus but is otherwise distinct and separate. Lymphatic channels are present throughout the substantia propria of the conjunctiva to the limbus where they form arcades. The limbus is one of the locations of stem cells and is a common site for the development of both squamous cell carcinoma and malignant melanoma. Lymphatic channels of the conjunctiva drain to the preauricular, parotid, and submental nodes (1,2).
Developmental Abnormalities of the Conjunctiva
Developmental abnormalities of the conjunctiva are infrequent. Occasionally, redundant, tortuous, dilated lymphatic vessels (lymphangiectasia) are present that may lead to symptoms because of dryness of elevated portions of the tissue or because of hemorrhage into the lymphatic spaces.
Episcleral osseous choristoma is due to embryonic rests of bone in the episcleral tissue, which may present as a stationary nodule often in the upper temporal quadrant of the conjunctiva or of the lower eyelid (27). The lesion generally consists of mature bone surrounded by mature fibrous tissue.
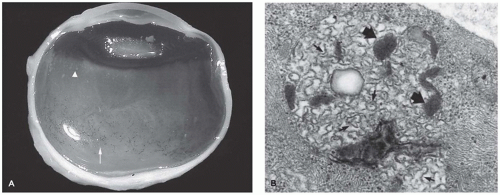
Limbal dermoid of the conjunctiva is a choristomatous nodule of dermal tissue, usually located at the limbus (Figure 11-8). The nodule may also be situated on the central corneal surface with only a minimal connection with the vascular system of the conjunctiva. The mass may obstruct the visual axis or alter the contour of the cornea and thereby cause amblyopia. The surface is nonkeratinizing squamous epithelium overlying dermal elements including mature fat. The lesion may involve the full thickness of the cornea and sclera but usually does not involve intraocular structures (Figure 11-9) (28). The lesion is usually solid without cystic elements as are found in cystic dermoid of the orbit (see below). Surgical removal may not result in normalization of corneal curvature.
Neuronal ceroid lipofuscinosis is a group of neurodegenerative diseases inherited in an autosomal recessive pattern that results in accumulation of lipopigments within cells and consequent disruption of cellular function (29). The conjunctiva is considered a convenient site for diagnostic biopsy. Intracellular “curvilinear bodies and fingerprint bodies” are found by examination with transmission electron microscopy (Figure 11-10) (see Chapters 5 and 10).
Inflammatory Conditions of the Conjunctiva
Microbial infections of the conjunctiva due to bacteria, viruses, and fungi occur commonly but are rarely biopsied. Trachoma remains a worldwide cause of significant blindness that results from infection with Chlamydia trachomatis. Early stages of the disease are characterized by an indolent follicular conjunctivitis. Late in the evolution of the disease, superficial scarring of the tarsal conjunctiva causes
contraction (entropion), forcing eyelashes (cilia) to contact and damage the superficial cornea. Blindness results from secondary corneal scarring and not from the conjunctival infection. Treatment of these advanced cases is surgical.
contraction (entropion), forcing eyelashes (cilia) to contact and damage the superficial cornea. Blindness results from secondary corneal scarring and not from the conjunctival infection. Treatment of these advanced cases is surgical.
Ligneous conjunctivitis is an accumulation of fibrin in the subepithelial space of mucous membranes throughout the body, caused by a systemic reduction in the levels of plasminogen (30). The condition usually presents in young females due to conjunctival symptoms (itching, burning, decreased vision) because of the presence of subconjunctival nodules composed of a woody-like accumulation of fibrin (31). The overlying epithelium is usually unremarkable, although signs of drying (epithelial thinning, reactive keratinization) may be present. Amorphous fibrin sometimes associated with an acute or chronic nongranulomatous inflammatory infiltrate may be present. Topical plasminogen concentrate has been used for treatment (32). Ligneous conjunctivitis may be associated with congenital occlusive hydrocephalus and juvenile colloid milium (31).
Melanocytic Abnormalities of the Conjunctiva
Melanosis of the conjunctiva is recognized clinically as hyperpigmentation of the conjunctiva without alteration of the surface contour of the conjunctiva. The lesion is present at birth or develops in early childhood as a yellow-brown to brownish black discoloration. There is a larger than average number of typical melanocytes and a higher than average accumulation of melanin in the conjunctival epithelium basal layers. This lesion is not a precursor for melanoma.
Melanosis of the episclera and scleral tissue is visible melanosis of the tissues deep to the conjunctiva, although the normal, transparent conjunctiva is visible as an area of slate gray discoloration. Heterochromia iridis and hyperpigmentation of the uveal tract may be present. Hyperpigmentation may extend to the meninges of the optic nerve and the brain. The contour of the overlying conjunctiva is not altered. The lesion may also present at birth and is generally stationary. A larger than average number of melanocytes is present as individual cells or small clusters interspersed in connective tissue. There is an increase in the number of melanocytes in the uveal tract; these melanocytes are larger than the indigenous melanocytes. The melanocytes are relatively hyperpigmented (Figure 11-11). This lesion is a risk factor for melanoma, which arises in the ipsilateral uveal tract or deep orbital tissues but not in the conjunctiva.
Nevus of Ota is a risk factor for ipsilateral uveal and orbital melanoma, particularly in Caucasians. In addition to hyperpigmentation of the eye (melanosis oculi) and orbit, there is hyperpigmentation of the eyelid and periorbital facial skin. Meningeal melanocytoma has also been associated with the nevus of Ota (33).
Melanocytic nevus of the conjunctiva is an accumulation of abnormal nevus cells in the region of the conjunctival epithelium. Early in life, in the junctional nevus stage, the nevus is a relatively well-demarcated area of the conjunctiva, which may not alter the surface contour and may be amelanotic or lightly pigmented. Conjunctival nevi are often associated with an anomalous development of the conjunctival epithelium, where the conjunctival epithelium is drawn into the substantia propria of the conjunctiva to form solid nests of squamous epithelium or cysts lined by squamous epithelium (Figure 11-12). The cysts may alter the surface contour of the conjunctiva, particularly if the cystic lining contains goblet cells or accessory lacrimal tissue that secretes into the lumen of the cysts. The melanocytes may be extensively pleomorphic, ranging from spindle-shaped cells to epithelioid cells. These atypical melanocytes may occur in the epithelium of the inclusion cysts giving the false impression of lymphatic spread of a melanoma. Clusters of melanocytic nevus cells may indent the lining of lymphatic channels also giving the appearance of lymphatic invasion.
Later in the natural history of a conjunctival melanocytic nevus, a dermal component develops (compound nevus). At puberty, there may be proliferation of melanocytes and increased density of pigmentation creating concern about the presence of a conjunctival melanoma. The nevus may appear to enlarge because of simultaneous proliferation of the squamous epithelial component of the inclusion cysts and increasing volume of the contents of the cyst (34). Irritation from drying of the elevated surface of the conjunctiva may also add to the impression of growth due to reactive inflammation and vascularization (35).
Melanoma of the conjunctiva may arise from a preexisting nevus or de novo even though primary acquired melanosis (PAM) with atypia does not generally occur in the pediatric age group but may be on the increase (18). A review of the international literature by Taban found 28 reported cases of conjunctival melanoma in individuals under the age of 15 years (36,37).
A conjunctival melanoma is an atypical proliferation of conjunctival melanocytes that has the potential of widespread metastasis and death. In the presence of a preexisting nevus or in the absence of histologic signs of a pre-existing
nevus, there is invasion of the substantia propria of the conjunctiva by malignant melanocytes. Features of malignancy include the presence of mitotic figures, atypical melanocytes in clusters, lack of the expected maturation with depth, and infiltrative growth at the deep margin. Cytologic features such as a spindle-shaped appearance of the cells is of no prognostic significance.
nevus, there is invasion of the substantia propria of the conjunctiva by malignant melanocytes. Features of malignancy include the presence of mitotic figures, atypical melanocytes in clusters, lack of the expected maturation with depth, and infiltrative growth at the deep margin. Cytologic features such as a spindle-shaped appearance of the cells is of no prognostic significance.
Squamous carcinoma of the conjunctiva rarely involves the pediatric age group, though it may be seen in xeroderma pigmentosum (38). The lesion may present as a papilloma, a gelatinous lesion, or as a leukoplakic mass of the conjunctiva. The initial histopathologic findings are atypia progressing to carcinoma in situ. The lesions are invasive if the underlying basement membrane is breached. Squamous cell carcinoma of the conjunctiva is usually indolent but may spread to regional nodes. Spindle cell and mucoepidermoid variants tend to be more aggressive and may invade the eye itself.
Surgical Procedures of the Conjunctiva
The repair process of the conjunctiva results in scarring that may restrict movement of the globe and may result in a cosmetically unacceptable appearance. Therefore, biopsies of the conjunctiva are generally limited in extent even in the presence of a suspected malignancy. Most biopsy sites of the conjunctiva are described in reference to the limbus (e.g., the specimen is from the 3 o’clock position of the right eye and extended nasally). Biopsies for the diagnosis of systemic disease (e.g., sarcoidosis) are often performed in the inferior fornix where there is a normal high density of resting lymphocytes. Surgical access to the eye (limbal incision, orbital biopsy) and for strabismus procedures is through the conjunctiva. Inclusion cysts may arise at a suture line if the wound margins are not precisely apposed. Inclusion cysts may be removed by a second procedure to improve cosmetic appearance. Conjunctival tissue is essentially a nonrenewable resource, making the surgeon hesitant to remove any more tissue than is absolutely necessary. When there is not enough conjunctiva to close a defect, amniotic membrane transplant tissue is often used to improve healing.
Histopathologic interpretation of a conjunctival biopsy is often difficult because this tissue, which may harbor potentially serious disease (melanoma, lymphoma, rhabdomyosarcoma), is delicate and can be easily crushed with the forceps; furthermore, the samples are usually small. Adding to the processing problem is the tendency of the conjunctival tissue to curl or deform, if immersion fixed in formalin. The optimal method of submission is to fix the conjunctiva after the tissue has been flattened on support media such as filter paper. Many tumors of significance arise at the limbus (the junction of cornea and sclera). Therefore, the tissue sections are usually oriented perpendicular to the limbus. Close communication with the surgeon is necessary to establish tissue margins of significance.
THE SCLERA AND EXTRAOCULAR MUSCLES
Structure of the Sclera and Extraocular Muscles
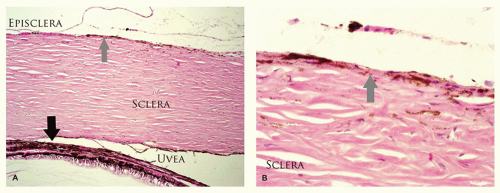
The sclera is formed by randomly oriented collagenous fibers that are more hydrated than the cornea and are therefore opaque. The sclera contains elastin fibers to accommodate changes in intravascular blood volume during systole and diastole. There are seven extraocular muscles that course through each orbit with various actions. The horizontal and cyclovertical muscles are responsible for ocular motility while the levator is responsible for eyelid excursion.
The sclera is relatively thin at the insertions of the four rectus muscles and the insertion of the superior oblique. The insertion of the inferior oblique is muscular rather than tendinous; therefore, there is no compensatory thinning. The sclera is breached by multiple ostia for the short and long posterior ciliary arteries posteriorly, the vortex veins near the equator, sensory nerves anteriorly, and multiple emissary channels carrying aqueous to the venous system near the limbus. Blood vessels supplying the anterior segment of the eye are located within the rectus muscle belly. There are two vessels in each rectus muscle, except the lateral rectus, which contains only one vessel. Disruption of more than four of these arteries during strabismus surgery can result in anterior segment ischemia.
THE CORNEA
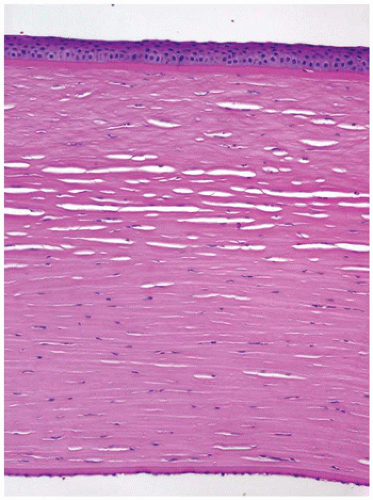
Structure of the Cornea
The cornea is the dominant element of the optical system of the eye, providing up to 80% of its refracting power. The power of the cornea is determined by its curvature, a feature that is highly conserved throughout life. The cornea is composed primarily of extracellular matrix (the corneal stroma). The principal component of the corneal stroma is uniform type I collagen bundles separated at a precise distance by highly specialized and uniform proteoglycans. The interfiber distance is determined by the degree of hydration of the proteoglycans (Figure 11-13). The anterior surface of the cornea is composed of extraordinarily homogeneous nonkeratinizing squamous epithelium. The corneal epithelium does not contain goblet cells or antigen-processing cells. The normal basement membrane of the corneal epithelium is not visible by light microscopy. It rests on an acellular band of dense type I collagen (Bowman membrane) that is found only in primates and birds. Bowman membrane probably functions in maintaining corneal curvature, does not thicken with age, and is not restored if damaged by pathologic processes (Figure 11-14). The corneal endothelium is derived from neural crest and not mesoderm and, therefore, is not stained by vascular endothelial markers (e.g., factor VIII); rather, it is S100 positive. The corneal endothelium produces a thick basement
membrane (Descemet membrane) that is not physically attached to the corneal stroma and thickens continuously throughout life (1,2).
membrane (Descemet membrane) that is not physically attached to the corneal stroma and thickens continuously throughout life (1,2).
Developmental Abnormalities of the Cornea
There is a small range of tolerance of corneal diameter in which the normal optical properties of the cornea function. Similarly, the radius of curvature must be precise in order to focus light on the retina.
Microcornea is a condition in which the cornea is less than 9 mm in diameter (horizontal limbus to limbus) at 1 year of age. Other anatomic abnormalities such as microphthalmos with cyst or persistent hyperplastic primary vitreous (PHPV) often coexist. Even nonsyndromic-associated abnormalities of the trabecular meshwork are likely to cause glaucoma. The cornea tissue generally has a normal histologic appearance except in the case of Peters anomaly (see following).
Megalocornea is a condition in which the cornea is greater than 11.5 mm in diameter (horizontal limbus to limbus) at 1 year of age. Developmental abnormalities are generally stationary and tend to be bilateral. Megalocornea may be associated with dislocated crystalline lens (ectopia lentis) and other abnormalities of the anterior segment. The structure of the cornea in megalocornea is generally normal.
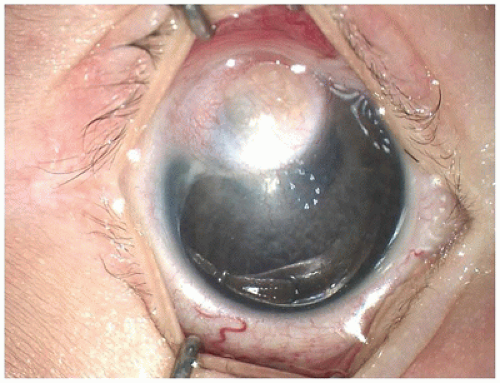
Peters anomaly most likely results from failure of separation of the cornea from the crystalline lens during embryonic development. In most cases the central posterior corneal stroma, Descemet membrane, and central corneal endothelium are absent. Rupture of Descemet membrane during forceps delivery or with corneal enlargement from glaucoma (buphthalmos with Haab striae) may appear similar histologically.
Herpes Simplex Keratitis
The herpes simplex virus initially affects the body as a systemic infectious disease with the cutaneous expression being a vesicular dermatitis. Live virus is retained in the gasserian ganglion and, for undetermined reasons, will periodically travel via sensory peripheral nerve to infect the corneal epithelium. Cytopathologic effects of the infected cells are seen as a linear, branching ulcer of the corneal epithelium (dendritic figure). With repeated episodes of infection, the corneal stroma becomes involved, not with direct viral infection but with lymphocytic infiltration, peripheral vascularization, and proteolysis of the extracellular matrix (discoid herpes keratitis, metaherpetic keratitis). The cornea can thin to the point of rupture. There may or may not be an intact epithelial covering. Bowman membrane ulcerates and Descemet membrane ruptures resulting in perforation of the cornea. A foreign body granulomatous response to the severed ends of
either or both Bowman membrane and Descemet membrane is a unique response in herpes simplex keratitis. There may be an extensive lymphocytic infiltrate in the keratoplasty specimen that may not be appreciated clinically. The degree of vascularization of the cornea is a risk factor for immunologic corneal rejection (39) (see Chapter 6).
either or both Bowman membrane and Descemet membrane is a unique response in herpes simplex keratitis. There may be an extensive lymphocytic infiltrate in the keratoplasty specimen that may not be appreciated clinically. The degree of vascularization of the cornea is a risk factor for immunologic corneal rejection (39) (see Chapter 6).
Acanthamoeba Keratitis
Acanthamoeba is a protozoa commonly found in soil and water. The organism can gain access to the cornea via microabrasions often associated with wearing contact lenses (40). The organism is neurotropic accounting for the extreme pain associated with infection. Organisms can be identified clinically by using confocal microscopy (41). The trophozoite form is motile and is able to spread extensively throughout the corneal stroma creating necrotizing keratitis and scarring. The encysted form can be identified throughout the cornea with most commonly used stains (Figure 11-15). There may be a limited inflammatory response because of treatment with anti-inflammatory drugs. Acanthamoeba is resistant to most forms of therapy (42). Occasionally, biopsy for diagnosis or penetrating keratoplasty for advanced stages of the infection is performed.
Dystrophic Conditions of the Cornea
Corneal dystrophies are metabolic abnormalities of the cornea that cause clinically detectable opacities. The prevalence of corneal dystrophy is extremely low. Most dystrophies are inherited in an autosomal dominant pattern (except macular corneal dystrophy, congenital hereditary endothelial dystrophy type 2 (CHED 2), lattice type 3, and gelatinous dystrophy, which are autosomal recessive), bilateral, symmetric, and progressive (at markedly variable rates) and recur in corneal grafted tissue. Even though the conditions are inherited, they generally do not progress sufficiently to be treated with penetrating keratoplasty in the pediatric age group except for CHED.
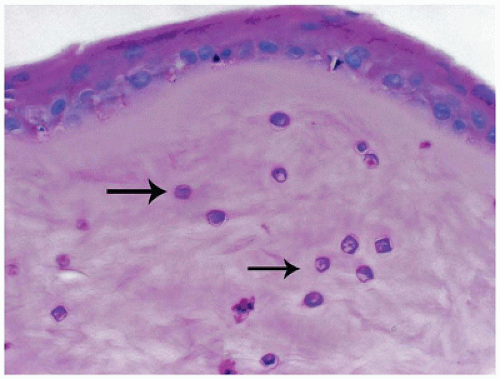 FIGURE 11-15 • Acanthamoeba keratitis. Multiple encysted Acanthamoeba organisms are present throughout the corneal stroma (periodic acid/Schiff stain, original magnification ×200). |
CHED is a congenital structural abnormality of the corneal stroma and endothelium that is inherited in both autosomal recessive (43,44) and autosomal dominant forms (45). The two forms are genetically distinct but both involve a region of chromosome 20 (46) with the recessive form mapping to 20p13 (47). Both corneas of an affected individual become thickened and opaque. An affected corneal collagen fiber diameter is nearly twice the normal diameter and is haphazardly arranged in a manner that limits transmission of light. Descemet membrane is often thin and the endothelium is abnormal. Treatment has traditionally been surgical.
Recently, multiple corneal dystrophies that were thought to be distinct clinically have been found to have a common genetic defect in the BIGH-3 gene (TGF-βI h3 gene located at 5q31) (48,49). This discovery has totally changed the classification of the affected corneal stromal dystrophies: Reis-Bücklers, lattice types I and III, granular, Avellino, and Thiel-Behnke corneal dystrophy (50).
Dystrophies of Bowman membrane (Reis-Bücklers and Thiel-Behnke dystrophies) occur very rarely. There is destruction of Bowman membrane possibly due to a protease produced in the corneal epithelium. Granular corneal dystrophy is an abnormality of protein metabolism of the corneal epithelial cells. Well-demarcated deposits occur initially in the anterior corneal stroma and progress to accumulate in deeper stromal layers. The intervening collagen is normal. Lattice corneal dystrophy type I is an accumulation of amyloid in the corneal stroma often in a linear pattern associated with the genetic abnormality at 5q31. Other subgroups of lattice corneal dystrophy involve other processes leading to amyloid deposition and are also rare. Avellino corneal dystrophy presents initially with features of granular corneal dystrophy and then progresses to develop features of lattice corneal dystrophy (“dystrophy” is an essential part of the name JDC). Studying affected patients living in Avellino, Italy, with this mixed clinical picture led to the discovery of the common genetic defect at 5q31 which is associated with multiple phenotypic expressions (51).
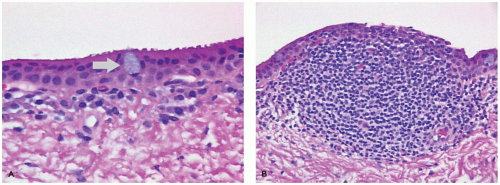
Map-dot-fingerprint (MDF) dystrophy, also known as anterior basement membrane dystrophy, is one of the most commonly occurring “dystrophies.” MDF dystrophy is characterized by excessive production of basement membrane material by the corneal epithelial cells (52). The epithelium is loosely adherent because of abnormal adhesive properties of the redundant basement membrane. Corneal abrasions tend to occur more frequently (recurrent erosion) (Figure 11-16). Secondary reactive degeneration of the Bowman membrane and anterior corneal stroma may occur if the abrasions are extensive, leading to superficial corneal opacification that is permanent. The condition rarely affects the pediatric age group and is treated with lubrication.
Macular corneal dystrophy is an abnormality of mucopolysaccharide production by corneal keratocytes (53). The condition is inherited in an autosomal recessive pattern and
most cases are caused by mutations in CHST6 gene. Unlike the other corneal dystrophies, there is a systemic abnormality in a subset of those with macular corneal dystrophy.
most cases are caused by mutations in CHST6 gene. Unlike the other corneal dystrophies, there is a systemic abnormality in a subset of those with macular corneal dystrophy.
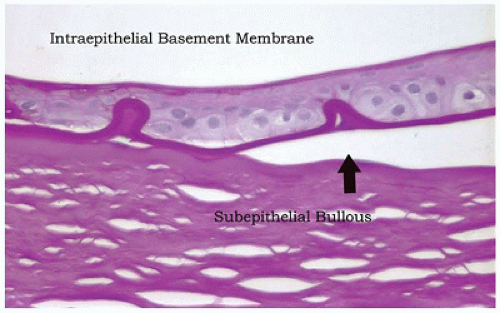 FIGURE 11-16 • Map-dot-fingerprint dystrophy. Epithelial cells have produced a defective basement membrane with abnormal adhesive characteristics. The epithelium has separated from the Bowman membrane to form a subepithelial bulla. The bullae are fragile and may rupture causing exposure of nerve endings and pain. The absence of epithelial cover is a risk factor for corneal infection (periodic acid/Schiff stain, original magnification ×100).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|