CHAPTER 30 The Colonic Phase of the Integrated Response to a Meal
OVERVIEW OF THE LARGE INTESTINE
The most distal segment of the gastrointestinal tract is called the large intestine, which is composed of the cecum; ascending, transverse, and descending portions of the colon; the rectum; and the anus (Fig. 30-1). The primary functions of the large intestine are to digest and absorb components of the meal that cannot be digested or absorbed more proximally, reabsorb the remaining fluid that was used during movement of the meal along the gastrointestinal tract, and store the waste products of the meal until they can conveniently be eliminated from the body. In fulfilling these functions, the large intestine uses characteristic motility patterns and expresses transport mechanisms that drive the absorption of fluid, electrolytes, and other solutes from the stool. The large intestine also contains a unique biological ecosystem consisting of many trillions of so-called commensal bacteria that engage in a life-long symbiotic relationship with their human host. These bacteria can metabolize components of the meal that are not digested by host enzymes and make their products available to the body via a process known as fermentation. Colonic bacteria also metabolize other endogenous substances such as bile acids and bilirubin, thereby influencing their disposition. There is emerging evidence that the colonic flora is critically involved in promoting development of the normal colonic epithelium and in stimulating its differentiated functions. In addition, these bacteria detoxify xenobiotics (substances originating outside the body, such as drugs) and protect the colonic epithelium from infection by invasive pathogens. Finally, the colon is both the recipient and the source of signals that allow it to communicate with other gastrointestinal segments to optimally integrate function. For example, when the stomach is filled with freshly masticated food, the presence of the meal triggers a long reflex arc that results in increased colonic motility (the gastrocolic reflex) and eventually evacuation of the colonic contents to make way for the residues of the next meal. Similarly, the presence of luminal contents in the colon causes the release of both endocrine and neurocrine mediators that slow propulsive motility and decrease electrolyte secretion in the small intestine. This negative-feedback mechanism matches the rate of delivery of colonic contents to the segment’s capacity to process and absorb the useful components. Details of the signals that mediate this crosstalk between the colon and other components of the gastrointestinal system are reviewed in the next section.
Signals That Regulate Colonic Function
The colon is relatively poorly supplied with cells that release bioactive peptides and other regulatory factors. Exceptions are enterochromaffin cells, which release 5-HT, and cells that synthesize peptide YY, so named because its sequence contains two adjacent tyrosine residues (Y is the single letter code for amino acids). Peptide YY is synthesized by enteroendocrine cells localized in the terminal ileum and colon and is released in response to lipid in the lumen. It decreases gastric emptying and intestinal propulsive motility. Peptide YY also reduces Cl− and thus fluid secretion by intestinal epithelial cells. Thus, peptide YY has been characterized as an “ileal brake” in that it is released if nutrients, especially fat, are not absorbed by the time that the meal reaches the terminal ileum and proximal part of the colon. By reducing propulsion of the intestinal contents, in part by limiting their fluidity and distention-induced motility, peptide YY provides more time for the meal to be retained in the small intestine, where its constituent nutrients can be digested and absorbed.
Patterns of Colonic Motility
Functional Anatomy of the Colonic Musculature
As in other segments of the intestine, the colon consists of functional layers with a columnar epithelium most closely opposed to the lumen, which is then underlaid by the lamina propria, serosa, and muscle layers. Similarly, the colonic mucosa is surrounded by continuous layers of circular muscle that can occlude the lumen. Indeed, at intervals, the circular muscle contracts to divide the colon into segments called haustra. These haustra are readily appreciated if the colon is viewed at laparotomy or by x-ray imaging as shown in Figure 30-2. The arrangement of the majority of the longitudinal muscle fibers, however, is distinct from that in the small intestine. Three nonoverlapping bands of longitudinal muscle, known as the taeniae coli, extend along the length of the colon.
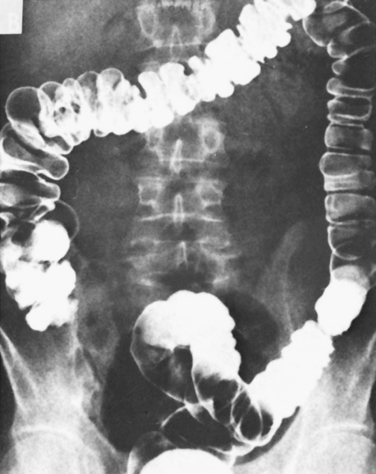
Figure 30-2 Radiograph showing a prominent haustral pattern in the colon of a normal individual.
(From Keats TE: An Atlas of Normal Roentgen Variants, 2nd ed. St Louis, Mosby–Year Book, 1979.)
Although the circular and longitudinal muscle layers of the colon are electrically coupled, this process is less efficient than in the small intestine. Thus, propulsive motility in the colon is less effective than in the small intestine. Activity of the enteric nervous system also provides for the segmenting contractions that form the haustra. Contents can be moved back and forward between haustra, which is a means of retarding passage of the colonic contents and maximizing their contact time with the epithelium. In contrast, when rapid propulsion is called for, the contractions forming the haustra relax, and the contour of the colon is smoothened.
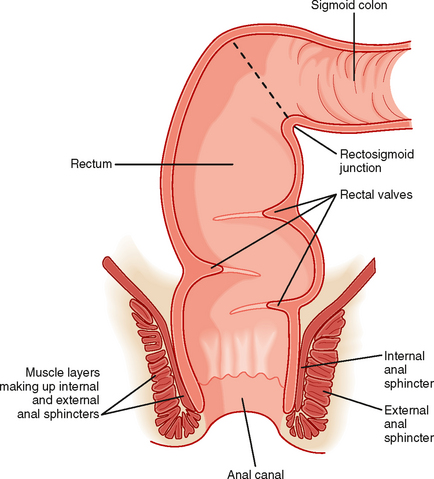
The colon terminates in the rectum, which is joined to the colon at an acute angle (the rectosigmoid junction) (Fig. 30-3). The rectum lacks circular muscle and is surrounded only by longitudinal muscle fibers. It is a reservoir wherein feces can be stored before defecation. Muscular contractions also form functional “valves” in the rectum that retard the movement of feces and are important in delaying the loss of feces until it is convenient, at least in adults. The rectum, in turn, joins the anal canal, distinguished by the fact that it is surrounded not only by smooth muscle but also by striated (skeletal) muscle. The combination of these muscle layers functionally accounts for two key sphincters that control the evacuation of solid waste and flatus from the body. The internal anal sphincter is composed of a thickened band of circular muscle, whereas the external anal sphincter is made up of three different striated muscle structures in the pelvic cavity that wrap around the anal canal. These latter muscles are distinctive because they maintain a significant level of basal tone and can be contracted further either voluntarily or reflexively when abdominal pressure increases abruptly (such as when lifting a heavy object).
Contraction of the smooth muscle layers in the proximal part of the colon is stimulated by vagal input, as well as by the enteric nervous system. On the other hand, the remainder of the colon is innervated by the pelvic nerves, which also control the caliber of the internal anal sphincter. Voluntary input from the spinal cord via branches of the pudendal nerves regulates contraction of the external anal sphincter and muscles of the pelvic floor. The ability to control these structures is learned during toilet training. This voluntary control distinguishes the anal canal from most of the gastrointestinal system, with the exception of the striated muscle in the esophagus that regulates swallowing.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree