The Chemical Basis of Life
BASIC CHEMISTRY
Elements and Compounds
Chemists use the term matter to describe in a general sense all of the materials or substances around us. Anything that has mass and occupies space is matter.
Substances are either elements or compounds. An element is said to be “pure” in the sense that it cannot be broken down or decomposed into two or more different substances. Carbon and oxygen are good examples of elements. In most living material, elements do not exist alone in their pure state. Instead, two or more elements are joined to form chemical combinations called compounds. Compounds can be broken down or decomposed into the elements that are contained within them. Water is a compound (H2O). It can be broken down into atoms of hydrogen and atoms of oxygen in a 2:1 ratio.
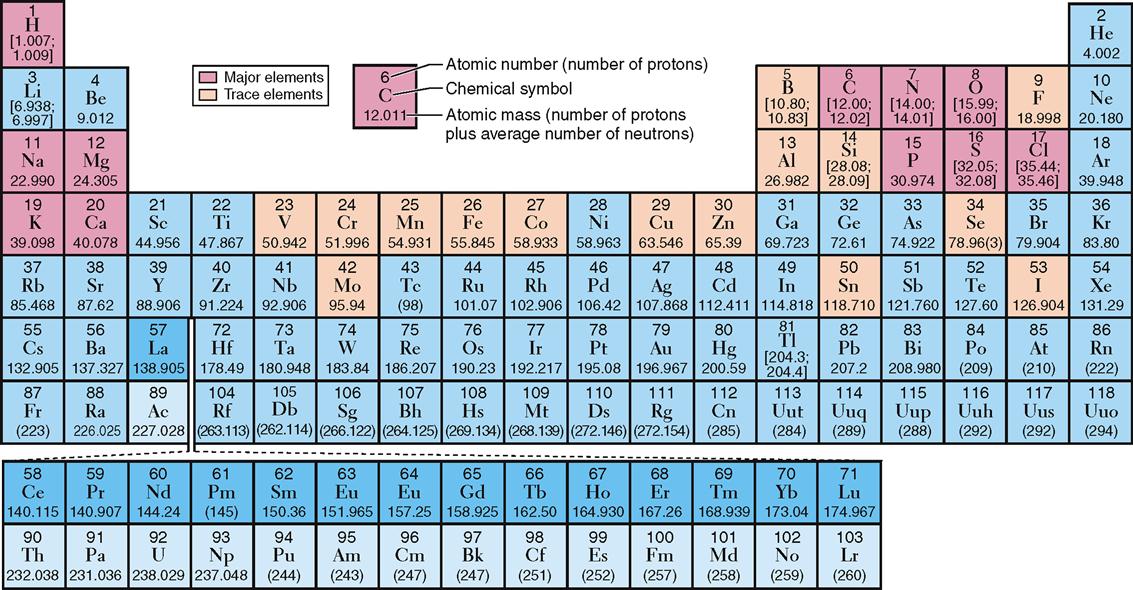
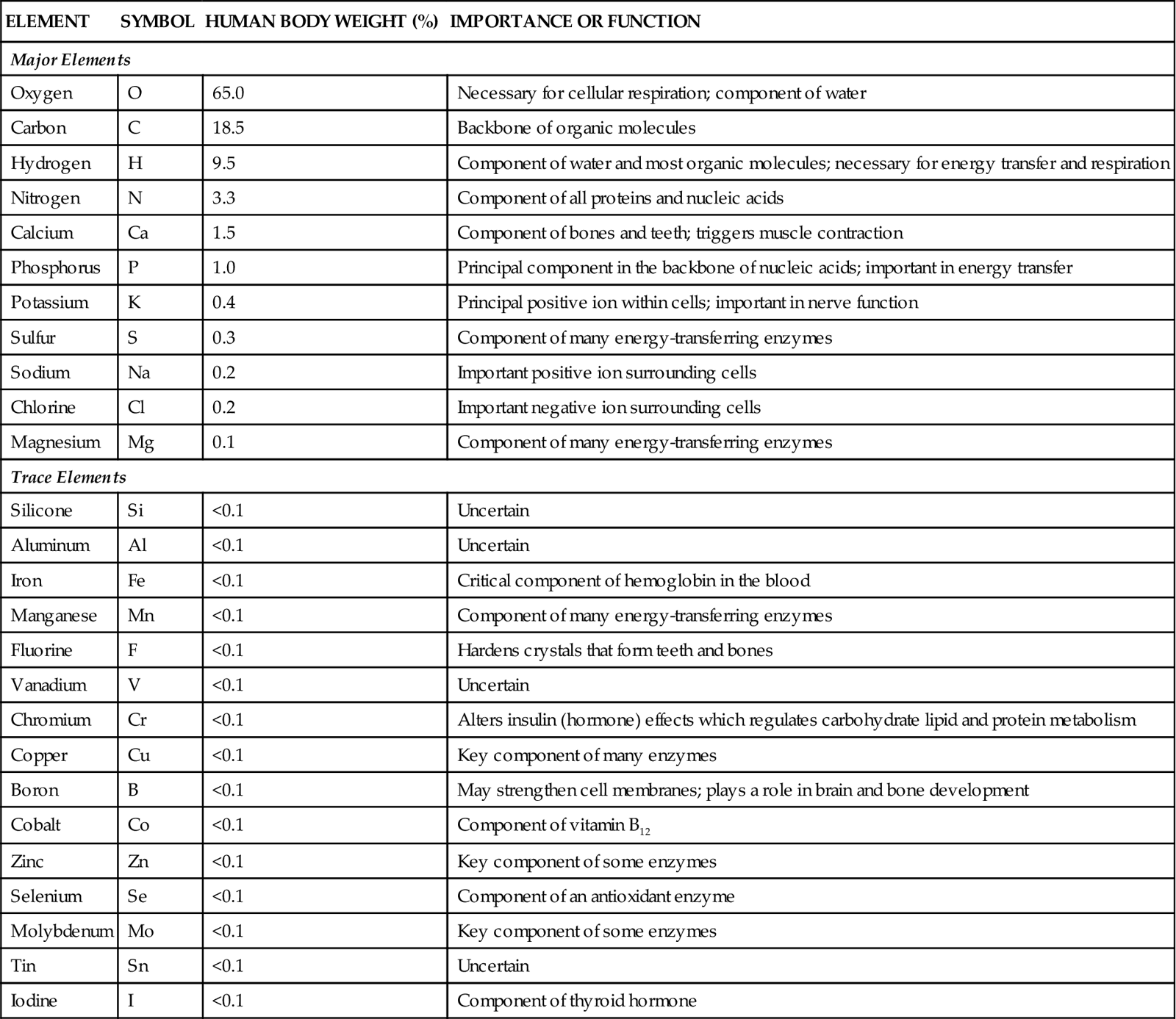
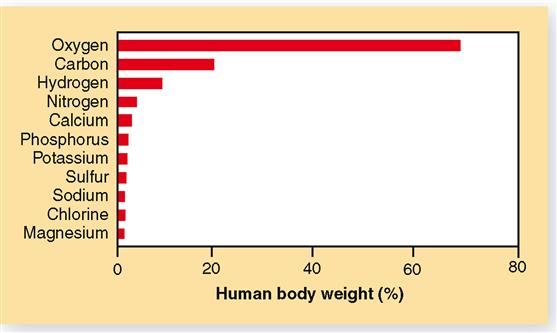
Other examples of elements include phosphorus, copper, and nitrogen (Figure 2-1). For convenience in writing chemical formulas and in other types of notation, chemists assign a symbol to each element, usually the first letter or two of the English or Latin name of the element: P, phosphorus; Cu, copper (Latin cuprum); N, nitrogen (see Figure 2-1). Note in Table 2-1 that 26 elements are listed as being present in the human body. Although all are important, 11 are called major elements. Four of these major elements—carbon, oxygen, hydrogen, and nitrogen—make up about 96% of the material in the human body (Figure 2-2). The 15 remaining elements are present in amounts that are less than 0.1% of body weight and are called trace elements. The unique “aliveness” of a living organism does not depend on a single element or mixture of elements but on the complexity, organization, and interrelationships of all elements required for life.
TABLE 2-1
| ELEMENT | SYMBOL | HUMAN BODY WEIGHT (%) | IMPORTANCE OR FUNCTION |
| Major Elements | |||
| Oxygen | O | 65.0 | Necessary for cellular respiration; component of water |
| Carbon | C | 18.5 | Backbone of organic molecules |
| Hydrogen | H | 9.5 | Component of water and most organic molecules; necessary for energy transfer and respiration |
| Nitrogen | N | 3.3 | Component of all proteins and nucleic acids |
| Calcium | Ca | 1.5 | Component of bones and teeth; triggers muscle contraction |
| Phosphorus | P | 1.0 | Principal component in the backbone of nucleic acids; important in energy transfer |
| Potassium | K | 0.4 | Principal positive ion within cells; important in nerve function |
| Sulfur | S | 0.3 | Component of many energy-transferring enzymes |
| Sodium | Na | 0.2 | Important positive ion surrounding cells |
| Chlorine | Cl | 0.2 | Important negative ion surrounding cells |
| Magnesium | Mg | 0.1 | Component of many energy-transferring enzymes |
| Trace Elements | |||
| Silicone | Si | <0.1 | Uncertain |
| Aluminum | Al | <0.1 | Uncertain |
| Iron | Fe | <0.1 | Critical component of hemoglobin in the blood |
| Manganese | Mn | <0.1 | Component of many energy-transferring enzymes |
| Fluorine | F | <0.1 | Hardens crystals that form teeth and bones |
| Vanadium | V | <0.1 | Uncertain |
| Chromium | Cr | <0.1 | Alters insulin (hormone) effects which regulates carbohydrate lipid and protein metabolism |
| Copper | Cu | <0.1 | Key component of many enzymes |
| Boron | B | <0.1 | May strengthen cell membranes; plays a role in brain and bone development |
| Cobalt | Co | <0.1 | Component of vitamin B12 |
| Zinc | Zn | <0.1 | Key component of some enzymes |
| Selenium | Se | <0.1 | Component of an antioxidant enzyme |
| Molybdenum | Mo | <0.1 | Key component of some enzymes |
| Tin | Sn | <0.1 | Uncertain |
| Iodine | I | <0.1 | Component of thyroid hormone |
Atoms
The most important of all chemical theories was advanced in 1805 by the English chemist John Dalton. He proposed the concept that matter is composed of atoms (from the Greek atomos, “indivisible”). His idea was revolutionary and yet simple—that all matter, regardless of the form it may assume (liquid, gas, or solid), is composed of units he called atoms.
Dalton conceived of atoms as solid, indivisible particles, and for about 100 years this was believed to be true. We now know that atoms are divisible into even smaller or subatomic particles, some of which exist in a “cloud” surrounding a dense central core called a nucleus. More than 100 million atoms of even very dense and heavy substances, if lined up, would measure barely an inch and would consist mostly of empty space! Our knowledge about the number and nature of subatomic particles and the central nucleus around which they move continues to grow as a result of ongoing research.
ATOMIC STRUCTURE
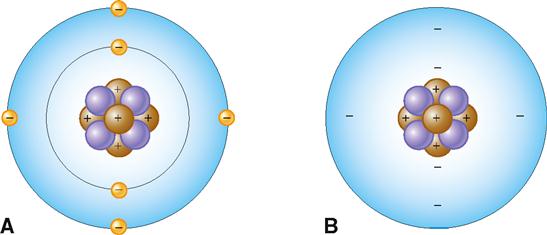
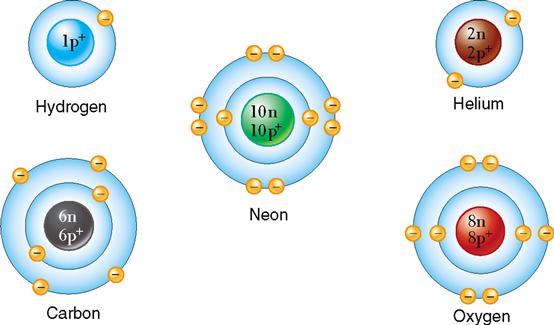
Atoms contain several different kinds of smaller or subatomic particles that are found in either a central nucleus or its surrounding “electron cloud” or “field.” Figure 2-3, A, shows an atomic model of carbon illustrating the most important types of subatomic particles:
Note that the carbon atom in Figure 2-3 has a central corelike nucleus. It is located deep inside the atom and is made up of six positively charged protons (p) and six uncharged neutrons (n). Note also that the nucleus is surrounded by a cloud or field of six negatively charged electrons (e). Because protons are positively charged and neutrons are neutral, the nucleus of an atom bears a positive electrical charge equal to the number of protons that are present in it. Electrons move around the atom’s nucleus in what can be represented as an electron cloud or field (Figure 2-3, B). The number of negatively charged electrons moving around an atom’s nucleus equals the number of positively charged protons in the nucleus. The opposite charges therefore cancel or neutralize each other, which means atoms are electrically neutral particles.
The so-called electron clouds are really just areas where the electrons are most likely to be found moving about rapidly. However, they can sometimes be visualized with a special form of microscope, as you can see in Figure 2-4.
ATOMIC NUMBER AND MASS NUMBER
Elements differ in their chemical and physical properties because of differences in the number of protons in their atomic nuclei. The number of protons in an atom’s nucleus, called its atomic number, is therefore critically important—it identifies the kind of element it is.
Look again at the elements important in living organisms listed in Table 2-1. Each element is identified by its symbol and atomic number. Hydrogen, for example, has an atomic number of 1; this means that all hydrogen atoms—and only hydrogen atoms—have one proton in their nucleus. All carbon atoms—and only carbon atoms—contain six protons and have an atomic number of 6. All oxygen atoms, and only oxygen atoms, have eight protons and an atomic number of 8. In short, each element is identified by its own unique number of protons, that is, by its own unique atomic number. If two atoms contain a different number of protons, they are different elements.
There are 92 elements that occur naturally on earth. Because each element is characterized by the number of protons in its atoms (atomic number), there are atoms that contain from 1 to 92 protons. Additional elements have been discovered as a result of sophisticated research in the area of particle physics.
The term mass number refers to the mass of a single atom. The mass number is sometimes called the atomic mass. It equals the number of protons plus the number of neutrons in the atom’s nucleus. The weight of electrons is, for practical purposes, negligible. Because protons and neutrons weigh almost exactly the same, the equation for determining mass number is as follows:
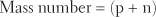
The largest naturally occurring atom is uranium. It has a mass number of 238, with a nucleus containing 92 protons and 146 neutrons. In contrast, hydrogen, which has only one proton and no neutrons in its nucleus, has a mass number of 1.
ENERGY LEVELS
The total number of electrons in an atom equals the number of protons in its nucleus (see Figure 2-3). These electrons are known to exist in regions surrounding the atom’s nucleus.
No single model of the atom sufficiently explains all we know about atomic structure. However, two simple models of atoms may be useful here to begin our discussion.
The cloud model suggests that any one electron cannot be exactly located at a specific point at any particular time. This concept is called a probability distribution and refers to the probability of finding an electron at any specific location outside the nucleus. Earlier models based on the work of a Danish physicist, Niels Bohr, who won the 1922 Nobel Prize in Physics for his groundbreaking contributions, suggested that electrons moved in regular patterns around the nucleus much like the planets in our solar system move around the sun. A simplified version of the Bohr model of the atom (see Figure 2-3, A) is perhaps most useful in visualizing the structure of atoms as they enter into chemical reactions.
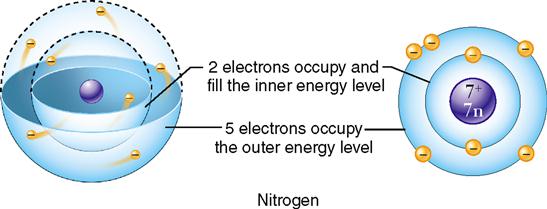
In the Bohr model, the electrons are shown in shells or concentric circles. The different shells show the relative distances of the electrons from the nucleus. The electrons surrounding the atom’s nucleus are seen in this model as existing in simple rings or shells. Each ring represents a different energy level, and each can hold only a certain maximum number of electrons (Figure 2-5). The number and arrangement of electrons orbiting in an atom’s energy levels are important because they determine whether the atom is chemically reactive.
In chemical reactions between atoms, it is the electrons in the outermost energy level that participate in the formation of chemical bonds. In each energy level, electrons tend to group in pairs. As a rule, an atom can be listed as chemically stable and unable to react with another atom if its outermost energy level has four pairs of electrons, or a total of eight. Such an atom is said to have a stable electron configuration. The pairing of electrons is important. If the outer energy level contains single, unpaired electrons, the atom will be chemically reactive. Atoms with fewer than eight electrons in the outer energy level will attempt to lose, gain, or share electrons with other atoms to achieve stability. This tendency is called the octet rule.
Consider an atom of oxygen, which has a total of six electrons in its outer energy level. As you can see in Figure 2-6, it has two unpaired electrons so it is two electrons short of satisfying the octet rule. Oxygen is likely to enter into chemical reactions to gain or share electrons with other atoms. By doing so, the oxygen atom will fill in its outer energy level and thus satisfy the octet rule.
The octet rule holds true except for atoms that are limited to a single energy level that is filled by a maximum of two electrons. For example, hydrogen has but one electron in its single energy level. It therefore has an incomplete energy level with an unpaired electron. The result is a highly reactive tendency of hydrogen to enter into many chemical reactions. Helium, however, has two electrons in its single energy level. Because this is the maximum number for this energy level, no chemical activity is possible, and no naturally occurring compound containing helium exists. Helium is an inert, or stable, element.
The atoms shown in Figure 2-6 illustrate several of the most important facts related to energy levels. Note that even in the hydrogen atom with its very basic structure, positive and negative charges balance. Hydrogen, carbon, and oxygen will react chemically because they do not satisfy the octet rule.
ISOTOPES
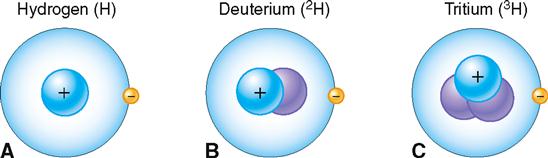
All atoms of the same element contain the same number of protons but do not necessarily contain the same number of neutrons. Isotopes of an element contain the same number of protons, but different numbers of neutrons.
Isotopes have the same basic chemical properties as any other atom of the same element, and they also have the same atomic number. However, because they have a different number of neutrons, they differ in mass number. Usually a hydrogen atom has only one proton and no neutrons (atomic number, 1; mass number, 1). Figure 2-7 illustrates this most common type of hydrogen and two of its isotopes. Note that the isotope of hydrogen called deuterium (2H) has one proton and one neutron (mass number, 2). Tritium (3H) is the isotope of hydrogen that has one proton and two neutrons (mass number, 3).
The term atomic weight refers to the average mass number for a particular element based on the typical proportion of different isotopes found in nature. Atomic weights are shown in the periodic table of elements illustrated in Figure 2-1, below each chemical symbol. Notice that the atomic weights for some elements, such as hydrogen and carbon, are listed as ranges instead of averages. For elements with isotope proportions that vary widely in nature, chemists find ranges more useful than averages.
The atomic nuclei of more than 99% of all carbon atoms in nature have six protons and six neutrons (atomic number, 6; mass number, 12). An important isotope of carbon has seven neutrons instead of six and is called carbon-13 (13C). Carbon-13 makes up about 1% of the world’s carbon atoms. The presence of carbon-13 in human tissues is useful in molecular studies of metabolic processes in the body. Another important carbon isotope has eight neutrons instead of six; it is called carbon-14 (14C). Carbon-14 is an example of a special type of isotope that is unstable and undergoes nuclear breakdown—it is designated as a radioactive isotope, or radioisotope. During breakdown, radioactive isotopes emit nuclear particles and radiation—a process called decay.
Attractions Between Atoms—Chemical Bonds
Interactions between two or more atoms occur largely as a result of activity between electrons in their outermost energy level. The result, called a chemical reaction, most often involves unpaired electrons.
Ultimately, in atoms with fewer or more than eight electrons in the outer energy level, reactions will occur that result in the loss, gain, or sharing of one atom’s unpaired electrons with those of another atom to satisfy the octet rule for both atoms. The result of such reactions between atoms is the formation of larger chemical structures such as crystals and molecules. For example, two atoms of oxygen can combine with one carbon atom to form molecular carbon dioxide, or CO2. If atoms of more than one element combine, the result, as defined earlier, is a compound. In other words, oxygen exists as a molecule (O2) and is an element. Water exists as a molecule (H2O) and is a compound. Reactions that hold atoms together do so by the formation of chemical bonds. There are two types of chemical bonds that unite atoms into larger structures: ionic (or electrovalent) bonds and covalent bonds.
IONIC BONDS
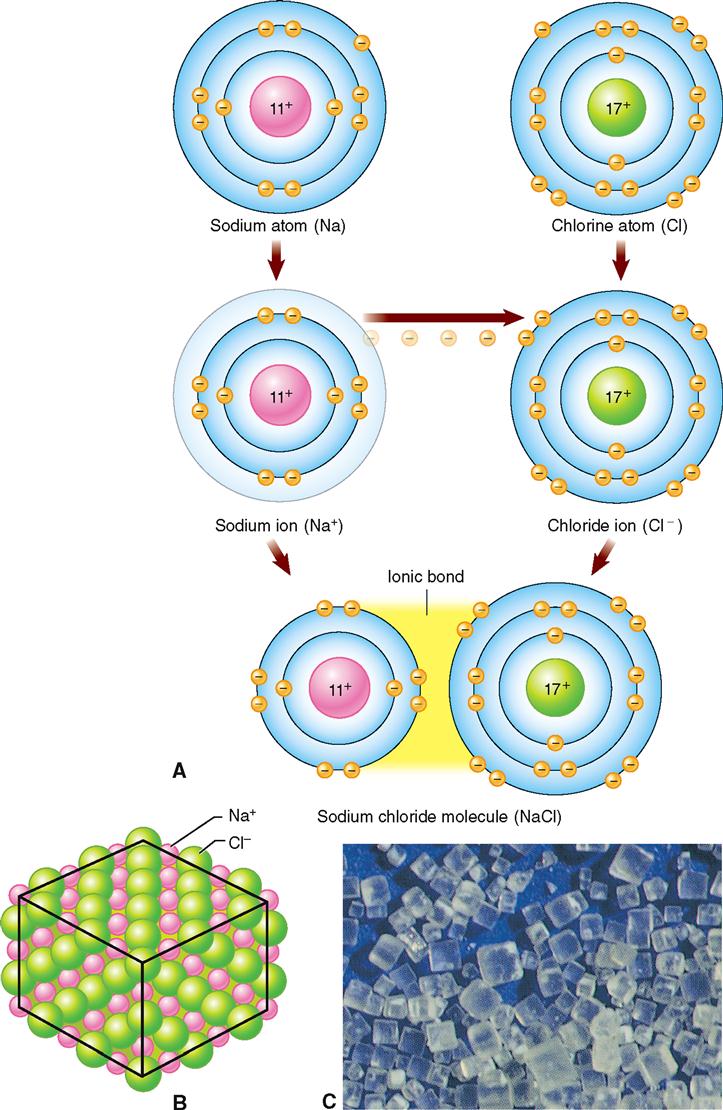
A chemical bond formed by the transfer of electrons from one atom to another is called an ionic, or electrovalent, bond. Such a bond occurs as a result of the attraction between atoms that have become electrically charged by the loss or gain of electrons. When dissolved in water (Figure 2-8), such atoms are separate into ions. It is important to remember that ions can be positively or negatively charged and that ions with opposite charges are attracted to each other.
Note in Figure 2-8, A, that in the outer energy level of the sodium atom there is a single unpaired electron. If this electron were “lost,” the outer ring would be stable because it would have a full outer octet (four pairs of electrons). The loss of the electron would result in the formation of a sodium ion (Na+) with a positive charge. This is because there is now one more proton (+) than electron (−).
The chlorine atom, in contrast, has one unpaired electron plus three paired electrons, or a total of seven electrons, in its outer energy level. By the addition of another electron, chlorine would satisfy the octet rule—its outer energy level would have a full complement of four paired electrons. The addition of another electron would result in the formation of a negatively charged chloride ion (Cl−).
Sodium transfers or donates its one unpaired electron to chlorine and becomes a positively charged sodium ion (Na+). Chlorine accepts the electron from sodium and pairs it with its one unpaired electron, thereby filling its outer energy level with the maximum of four electron pairs and becoming a negatively charged chloride ion (Cl−). The positively charged sodium ion (Na+) is attracted to the negatively charged chloride ion (Cl−), and the formation of NaCl crystals, ordinary table salt, results. This process illustrates ionic or electrovalent bonding.
The electron transfer changed the two atoms of the elements sodium and chlorine into ions. An ionic bond is simply the strong electrostatic force that binds the positively and negatively charged ions together in a crystal.
COVALENT BONDS
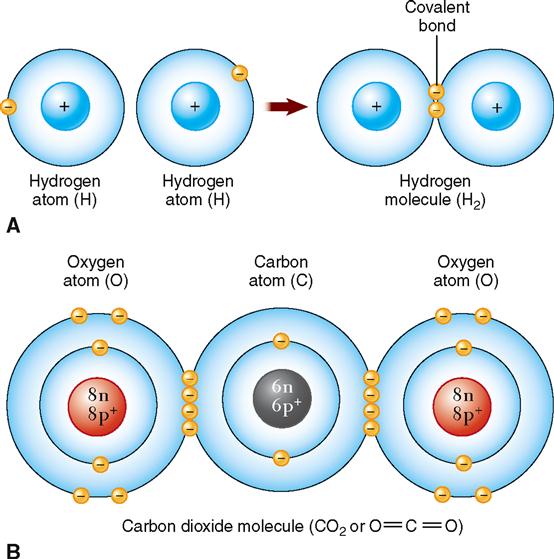
Just as atoms can be held together in crystals by ionic bonds formed when atoms gain or lose electrons, atoms can also be bonded together into molecules by sharing electrons. A chemical bond formed by the sharing of one or more pairs of electrons between the outer energy levels of two atoms is called a covalent bond. This type of chemical bonding is of great significance in physiology.
The major elements of the body (carbon, oxygen, hydrogen, and nitrogen) almost always share electrons to form covalent bonds. For example, if two atoms of hydrogen are bound together by the sharing of one electron pair, a single covalent bond is said to exist, and a molecule of hydrogen gas results (Figure 2-9, A). Covalent bonds that bind atoms together by sharing two pairs of electrons are called double bonds (Figure 2-9, B). The example shown illustrates two atoms of oxygen, each sharing two electrons with a carbon atom to acquire a complete outer energy level of eight electrons and thus satisfy the octet rule. A molecule of carbon dioxide results.
Attractions Between Molecules
HYDROGEN BONDS
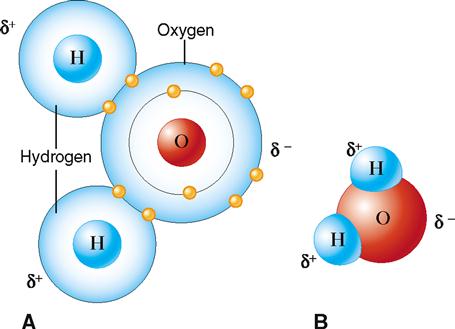
In addition to ionic and covalent bonds, another type of attractive force, called a hydrogen bond, can exist between biologically important molecules. Hydrogen bonds are much weaker forces than ionic or covalent bonds because they require less energy to break. Although an individual hydrogen bond is weak, large numbers of these bonds can collectively exert a strong attractive force. Instead of forming as a result of transfer or sharing of electrons between atoms, hydrogen bonds result from unequal charge distribution on a molecule. Such molecules are said to be polar.
Water is a good example of a polar molecule. Note in Figure 2-10 that although an atom of water is electrically neutral (the number of negative charges equals the number of positive charges), it has a partial positive charge (the hydrogen side) and a partial negative charge (the oxygen side). That is, the water molecule has a positive pole and a negative pole. The partial charges result from the electrons having a higher probability of being found nearer the highly positive oxygen nucleus than either hydrogen nucleus. That is, the electrons are not shared equally within the molecule. Thus water is said to be “polar” because it has regions with different partial charges. Hydrogen bonds serve to weakly attach the partially negative (oxygen) side of one water molecule to the partially positive (hydrogen) side of an adjacent water molecule.
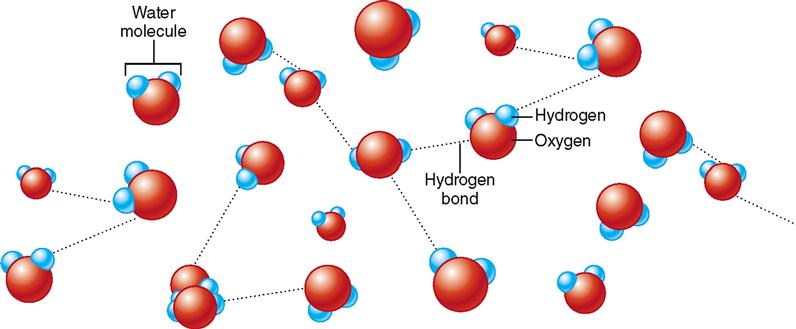
Figure 2-11 illustrates hydrogen bonding between water molecules. Depending on how many of these hydrogen bonds are intact at one instant, the water may be either liquid (few bonds) or solid (many bonds). If the water molecules are too far apart to form any hydrogen bonds, then the water is a gas, such as steam.
Hydrogen bonds form only between H atoms that are covalently bonded to an oxygen, nitrogen, or fluorine atom. In water molecules H is bonded to O, thus producing the polarity that permits the formation of hydrogen bonds between water molecules.
The ability of water molecules to form hydrogen bonds between molecules accounts for many of the unique properties of water that make it an ideal medium for the chemistry of life. Hydrogen bonds are also important in maintaining the three-dimensional structure of proteins and nucleic acids, also described later in the chapter.
OTHER WEAK ATTRACTIONS
Other weak attractions sometimes attract molecules to each other, even if only temporarily. Shifts in the locations of electrons within each molecule result in fleeting changes in the partial electrical charge of some regions of the molecule. This change in electrical charge may result in attraction to oppositely charged regions of another molecule. In the scope of our course, however, we will not concern ourselves with the different varieties of weak attractions or how they are produced. It is important to know only that they exist and sometimes play an underlying role in holding the material of the body together.
Chemical Reactions
Chemical reactions involve interactions between atoms and molecules, which in turn involves the formation or breaking of chemical bonds. Three basic types of chemical reactions that you will learn to recognize as you study physiology are the following:
To the chemist, reactions can be symbolized by variations on a simple formula. In synthesis reactions, two or more substances called reactants combine to form a different, more complex substance called a product. Synthesis literally means “putting together.” The process can be summarized by the following formula:

Synthesis reactions result in the formation of new bonds, and energy is required for the reaction to occur and the new product to form. Many such reactions occur in the body. Every cell, for example, combines amino acid molecules as reactants to form complex protein compounds as products. The ability of the body to synthesize new tissue in wound repair is a good example of this type of reaction.
Decomposition reactions result in the breakdown of a complex substance into two or more simpler substances. In this type of reaction, chemical bonds are broken and energy is released. Energy can be released in the form of heat, or it can be captured for storage and future use. Decomposition reactions can be summarized by the following formula:
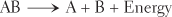
Decomposition reactions occur when a complex nutrient is broken down in a cell to release energy for other cellular functions. The products of such a reaction are ultimately waste products. Decomposition and synthesis are opposites. Synthesis builds up; decomposition breaks down. Synthesis forms chemical bonds; decomposition breaks chemical bonds. Decomposition and synthesis reactions are often coupled with one another in such a way that the energy released by a decomposition reaction can be used to drive a synthesis reaction.
The nature of exchange reactions permits two different reactants to exchange components and, as a result, form two new products. An exchange reaction is often symbolized by the following formula:
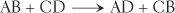
Exchange reactions break down, or decompose, two compounds and, in exchange, synthesize two new compounds. Certain exchange reactions take place in the blood. One example is the reaction between lactic acid and sodium bicarbonate. The decomposition of both substances is exchanged for the synthesis of sodium lactate and carbonic acid. These changes can be seen more easily in the following equation:
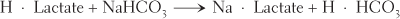
The formula H · Lactate represents lactic acid; NaHCO3 is the formula for sodium bicarbonate; Na · Lactate represents sodium lactate; and H · HCO3 represents carbonic acid.
Reversible reactions, as the name suggests, proceed in both directions. A great many synthesis, decomposition, or exchange reactions are reversible, and a number of them are cited in later chapters of this book. An arrow pointing in both directions is used to denote a reversible reaction:

METABOLISM
The term metabolism is used to describe all the chemical reactions that occur in body cells. The important topics of nutrition and metabolism are discussed fully in Chapter 30. Nutrition and metabolism are described together because the total of all the chemical reactions or metabolic activity occurring in cells is associated with the use the body makes of foods after they have been digested, absorbed, and circulated to cells. The terms catabolism and anabolism are used to describe the two major types of metabolic activity.
Catabolism describes chemical reactions that break down larger food molecules into smaller chemical units and, in so doing, often release energy. The release of energy is related to the disruption of chemical bonds. This breakdown of bonds in the chemical compounds contained in the foods and beverages that we consume provides the energy to power all of our activities.
Anabolism involves the many chemical reactions that build larger and more complex chemical molecules from smaller subunits (Figure 2-12). Anabolic chemical reactions require energy—energy most often made available by the breakdown of adenosine triphosphate (ATP).
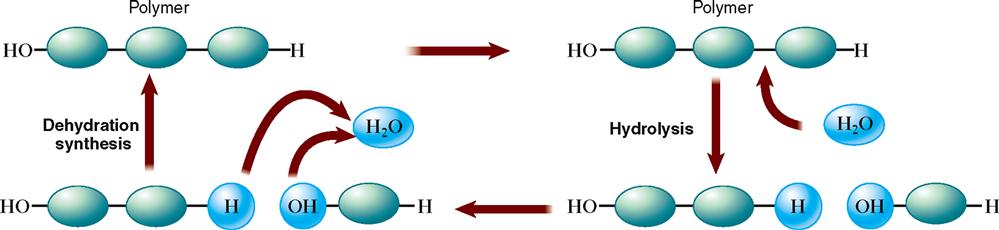
Catabolism
Catabolism consists of chemical reactions that not only break down relatively complex compounds into simpler ones but also release energy from them. This breakdown process represents a type of chemical reaction called hydrolysis (see Figure 2-12). As a result of hydrolysis occurring during catabolism, a water molecule is added to break a larger compound into smaller subunits. For example, hydrolysis of a fat molecule breaks it down into its subunits—glycerol and fatty acid molecules; a disaccharide such as sucrose breaks down into its monosaccharide subunits—glucose and fructose; and the subunits of protein hydrolysis are amino acids.
Ultimately, catabolic reactions will further degrade these building blocks of food compounds—glycerol, fatty acids, monosaccharides, and amino acids—into the end products carbon dioxide, water, and other waste products. During this process, energy is released. Some of the energy released by catabolism is heat energy, the heat that keeps our bodies warm. However, more than half the energy released is immediately recaptured and transferred to a molecule called ATP, which transfers the energy to cell components that need it to do work. ATP is discussed in more detail later in this chapter.
Anabolism
Anabolism is the term used to describe chemical reactions that join simple molecules together to form more complex biomolecules—notably, carbohydrates, lipids, proteins, and nucleic acids. Literally thousands of anabolic reactions take place continually in the body. The type of chemical reaction responsible for this joining together of smaller units to form larger molecules is called condensation or dehydration synthesis (see Figure 2-12). It is a key reaction during anabolism. As a result of dehydration synthesis, water is removed as smaller subunits are fused together.
Anabolism requires energy, which is transferred from ATP molecules. Anabolic reactions use energy to join monosaccharide units to form larger carbohydrates, fuse amino acids into peptide chains, and form fat molecules from glycerol and fatty acid subunits.
ORGANIC AND INORGANIC COMPOUNDS
In living organisms, there are two kinds of compounds: organic and inorganic. Organic compounds are generally defined as compounds composed of molecules that contain carbon–carbon (C–C) covalent bonds or carbon–hydrogen (C–H) covalent bonds—or both kinds of bonds. Few inorganic compounds have carbon atoms in them, and none have C–C or C–H bonds. Organic molecules are generally larger and more complex than inorganic molecules.
The term functional groups
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree