24.1 PRESERVATION OF VIRUS INFECTIVITY
There are several practical situations in which there is a need to preserve virus infectivity. A prime example is during the storage and distribution of live virus vaccines (Sections 25.2 and 25.5), when there is a need to retain the potency of the vaccine. A second example is during the transport and storage of blood, feces, saliva, and other specimens from human and animal patients prior to testing in a diagnostic virology laboratory. Last, but by no means least, virologists need to preserve the infectivity of the viruses that they work with.
24.2 DESTRUCTION OF VIRUS INFECTIVITY
There are many situations in which there is a requirement to destroy virus infectivity. Procedures to minimize the transmission of pathogenic viruses (and other micro-organisms) in hospitals and other health care facilities include the disinfection of surfaces and the sterilization of equipment and materials. A good example of a situation requiring rigorous action is an outbreak of winter vomiting disease caused by a norovirus; patients’ vomit contains significant quantities of virus, which must be inactivated if the outbreak is to be brought under control.
Inactivated (killed) virus vaccines (Section 25.3) are made from preparations of virulent viruses, so it is essential that all infectivity is destroyed. Blood products, such as clotting factors for hemophiliacs and immunoglobulins, are treated to destroy any viruses (e.g. HIV, hepatitis viruses) that might be present in the donated blood. Continuing the theme of human and animal health, water supplies that are free from pathogenic viruses (and other micro-organisms) are of fundamental importance, so water treatment procedures must include processes that destroy these agents. The same applies to the treatment of swimming pool water.
The processes for production of cheese, yogurt, and other dairy products utilize lactic acid bacteria to ferment the milk. There are many phages that infect these bacteria, and a phage infection during a production process can result in delay or failure of the fermentation, or in a poor quality product. To minimize the risk, procedures are required for treatment of the milk or whey to inactivate any phages present, and for the disinfection of the factory environment.
Finally, virologists need supplies of pipettes, cell culture vessels, and other laboratory materials that are guaranteed free from viruses (and other micro-organisms), then after these materials have been used any viruses present must be inactivated before disposal.
24.3 INACTIVATION TARGETS IN VIRIONS
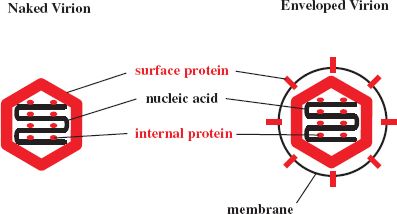
The infectivity of a virion can be destroyed by the alteration or removal of its nucleic acid and/or one or more of its proteins (Figure 24.1). A protein molecule may be altered by the induction of a conformational change or, more drastically, by the breaking of covalent bonds, such as peptide bonds and disulfide bonds.
Figure 24.1 Inactivation targets in virions. Infectivity of a virion may be destroyed by damage to a nucleic acid, a protein, and/or a lipid membrane. Alteration of a surface protein might prevent a virion from attaching to its host cell and/or from entering the cell. Stripping the envelope from an enveloped virion removes the surface proteins and achieves the same outcome. Alteration of internal proteins can destroy properties, such as enzyme activities, essential for the replication of the virus.
24.4 INACTIVATION KINETICS
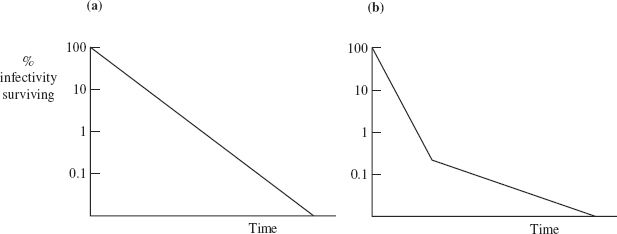
The rate at which a virus loses infectivity under defined conditions (for example, at a particular temperature and pH value) can be determined by incubating aliquots of the virus preparation under those conditions. At various time intervals the surviving infectivity in an aliquot is determined in an infectivity assay appropriate for the virus (Section 2.8). The logarithm of the percentage infectivity surviving is plotted against time (Figure 24.2).
In some circumstances infectivity is lost at a constant rate until no further infectivity can be detected (Figure 24.2(a)). The slope of the line is a measure of the rate of infectivity loss. Sometimes there is a change in the rate of infectivity loss after most (usually more than 99%) of the infectivity has been destroyed (Figure 24.2(b)). It is not entirely clear why a small fraction of some virus preparations loses infectivity at a slower rate. It has been suggested that this fraction might contain virions that have a higher degree of resistance to the inactivating factor and/or that the preparation might contain virion clumps, with virions at the centers of the clumps having some protection.
24.5 AGENTS THAT INACTIVATE VIRUS INFECTIVITY
24.5.1 Physical agents
24.5.1.a Temperature
All infective viruses lose infectivity over time, the rate of infectivity loss being highly dependent on temperature. There is a lot of variability between viruses in the rates at which they are inactivated. In general, naked viruses are more stable than enveloped viruses; most naked viruses lose little infectivity after several hours at 37 °C, whereas most enveloped viruses lose significant infectivity under the same conditions. This generalization is broad, as there may be significant differences in stability between closely related viruses. Under dry conditions varicella-zoster virus survives well, while the related herpes simplex viruses survive poorly.
At temperatures below –35 °C most viruses lose infectivity very slowly, so for long-term storage of viruses they are placed in freezers or in liquid nitrogen. Preservation of infectivity may be enhanced by storing virus preparations in a freeze-dried form, rather than as aqueous suspensions. Moving to higher temperatures, most viruses are completely inactivated within 30 minutes at 60 °C or within a few seconds at 100 °C.
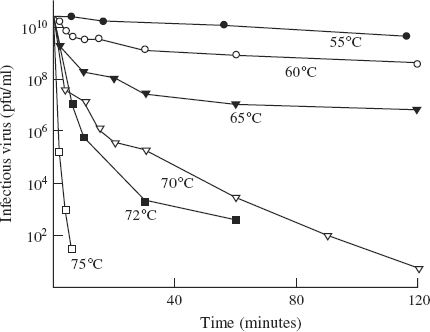
Some of the bacteriophages of lactic acid bacteria that cause problems in the dairy industry are quite heat resistant, and some infectivity may survive the heat treatments used in the decontamination procedures (Figure 24.3).
Figure 24.3 Inactivation of Lactococcus lactis phage P008 infectivity in a broth at temperatures between 55 and 75 °C.
Source: Data from Müller-Merbach et al. (2005) International Dairy Journal, 15, 777. Reproduced by permission of Elsevier Limited and the authors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree