25.1 INTRODUCTION TO VIRUS VACCINES
In this chapter we discuss one of the major applications of virology: the development and use of vaccines against virus diseases in humans and animals.
The term vaccination is derived from the Latin word vacca, meaning cow. This is because the original procedure involved the inoculation of material from cowpox lesions into healthy people. Edward Jenner tried the procedure first in 1796 after he noticed that the faces of most milkmaids were unmarked by pocks; this was because milkmaids rarely contracted smallpox. They did, however, commonly contract cowpox, so Jenner inoculated material from a milkmaid’s cowpox lesion into the arm of an 8-year-old boy. Six weeks later the boy was inoculated with material from a smallpox scab; he remained healthy. The immune response against cowpox virus had protected against smallpox virus, the protection resulting from related antigens in the two viruses.
A vaccine, therefore, contains material intended to induce an immune response, and this may involve both B cells (which develop into antibody-producing cells) and T cells (responsible for cell-mediated immunity). Both arms of adaptive immunity can be important for providing protection against a virus infection (Section 9.2.2).
The purpose of most viral vaccines is to induce long-term immunity against the virus by establishing B cell and T cell memory that will be triggered if the virus ever invades the body. In order to establish strong immunological memory there is a requirement for vaccines that induce vigorous immune responses; in other words, highly immunogenic virus materials are required.
Vaccines are in use to protect against diseases caused by many viruses such as polio, rubella, rabies, and foot and mouth disease. Major achievements resulting from vaccine use include the eradication of smallpox in humans and of rinderpest in cattle. This chapter will describe the various categories of virus vaccine that are in medical and veterinary use, and will outline some aspects of their manufacture.
Effective vaccines have yet to be developed against many other viruses, including HIV-1, hepatitis C, Ebola, and the herpes simplex viruses. Those involved in virus vaccine research face many difficulties, such as multiple antigenic variants of target viruses and the requirements for high standards of safety. Some vaccines that have been developed have not been accepted for widespread use because of safety concerns. The great need for new vaccines has spawned attempts to produce novel categories of vaccine, such as replication-defective viruses, peptide vaccines, and DNA vaccines, and some of these will be introduced in this chapter.
25.2 LIVE ATTENUATED VIRUS VACCINES
A live attenuated vaccine contains a mutant strain of a virus that has been derived from a wild-type virulent strain. Vaccines of this type have a number of advantages over most other types of vaccine. One advantage is that there are increasing amounts of virus antigen in the body as the virus replicates. Another is that a wide-ranging immune response is induced that involves B cells, CD4 T cells, and CD8 T cells.
There are two properties that the virus for an attenuated vaccine must possess. First, its antigens must be identical, or very similar, to those of the wild-type virus, so that an immune response against the vaccine virus provides protection from infection with the wild-type virus. Second, the virulence of the wild-type virus must have been attenuated; in other words, the vaccine virus must have little or no virulence.
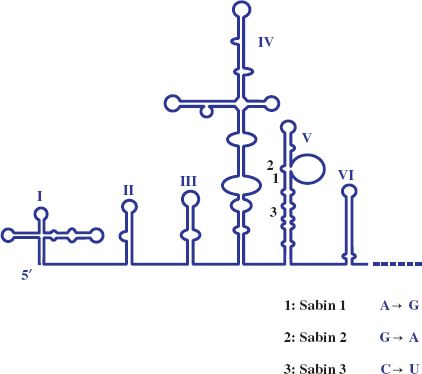
Most attenuated virus strains have been derived by “hit and miss” procedures such as repeated passage of wild-type virus in cells unrelated to the normal host. The vaccine strains of the three serotypes of poliovirus (Chapter 14) were derived from wild-type strains by passage in monkeys and in monkey kidney cell cultures. Albert Sabin did this pioneering work and the procedure he used to derive one of the attenuated strains is depicted in Figure 25.1. Poliovirus attenuation involves the loss of the ability of the virus to infect neurons (i.e., the loss of neurovirulence). The mutations responsible for attenuation of the three serotypes are now known and some of them are shown in Figure 25.2.
Figure 25.1 Derivation of attenuated poliovirus strain (Sabin type 1) from wild-type poliovirus strain (Mahoney 1).
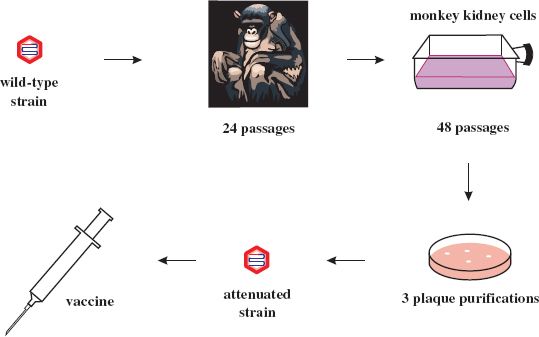
Figure 25.2 5′ end of poliovirus RNA with expanded view of domain V. For each of the three Sabin strains a mutation in domain V that contributes to the attenuation of neurovirulence is indicated.
Other attenuated vaccines that have been developed using a similar approach include vaccines for mumps, measles, rubella, yellow fever, canine parvovirus, and canine distemper. The attenuated strains of the first three of these, now combined in the MMR vaccine, were all developed by Maurice Hilleman. The attenuated mumps virus is called Jeryl Lynn after his daughter, from whom the wild-type virus was isolated.
Attenuated vaccines have been developed in response to the huge burden of disease and death caused by rotaviruses (Chapter 13). The vaccines contain reassortant rotaviruses (Section 21.3.3.c). One vaccine has genes derived from different human viruses, while another has some genes derived from a human virus and some from a bovine virus. These rotavirus vaccines are given by mouth.
Other approaches to the development of attenuated virus vaccines include the following.
- Cold-adapted virus strains. These are virus strains with reduced virulence that have been derived by incubating virus-infected cell cultures at temperatures below the optimum for virus replication. The strains are unable to replicate at normal body temperature, but can replicate at temperatures in the upper respiratory tract. Cold-adapted strains are used in influenza and respiratory syncytial virus vaccines, but their use is restricted because of the risk of side effects. The cold-adapted influenza vaccine is not used in elderly, pregnant, or immunocompromised persons and the respiratory syncytial virus vaccine is not used in children.
- Reverse genetics (Sections 14.6.1 and 15.6). The power of reverse genetics has been used to develop a respiratory syncytial virus vaccine by engineering into the virus genome mutations that attenuate virulence.
One of the risks in using a live vaccine is that, during virus replication, nucleotide substitutions might occur, resulting in reversion to virulence. Poliovirus is an RNA virus with a high mutation rate. When a vaccine strain of poliovirus replicates in the gut there may be nucleotide substitutions when it replicates its genome, or it may undergo recombination with a wild-type strain of poliovirus (Section 21.3.3.b). Either event may result in a virulent strain of poliovirus which can infect susceptible individuals, resulting in poliomyelitis for some. Because of these risks many countries switched from using attenuated polio vaccine to inactivated vaccine.
Each batch of a live virus vaccine is subjected to extensive quality control tests. These include testing for the presence of every known virus that could be a contaminant, and testing for neurovirulence in animals.
25.3 INACTIVATED VIRUS VACCINES
Inactivated, or killed, virus vaccines are made by mass-producing the virulent virus and then inactivating the infectivity, usually by treatment with a chemical such as β-propriolactone, ethylene oxide, or formaldehyde (Section 24.5.2.c). The trick lies in finding the combination of chemical concentration and reaction time that completely inactivates the virus, but leaves its antigens sufficiently unchanged that they can still stimulate a protective immune response.
Jonas Salk developed a treatment for poliovirus that led to the development of the vaccine that bears his name. After treatment with formaldehyde the inactivated virions (a mixture of the three serotypes of poliovirus) are adsorbed onto an adjuvant consisting of aluminum hydroxide or aluminum phosphate. The adjuvant enhances the immunogenicity of the vaccine. Other examples of killed virus vaccines are those containing inactivated virions of influenza, hepatitis A, and foot and mouth disease viruses.
Because the virus used to produce an inactivated vaccine is a virulent strain, it is vital that 100% of the infectivity is destroyed in the production process. As discussed in Section 24.4, there are some situations in which a small proportion of virions is inactivated at a slower rate. It is essential that the kinetics of virus inactivation are understood and that an inactivation procedure is developed that guarantees 100% inactivation.
The need for complete inactivation was underlined in the US in 1955 when four million doses of inadequately inactivated Salk vaccine were inoculated into children. Amongst the vaccinees there were 204 cases of paralytic polio and 11 deaths. A similar problem occurred when there were outbreaks of foot and mouth disease in France and the UK in the period 1979–81 after batches of foot and mouth disease vaccine had been inadequately inactivated.
25.4 VIRION SUBUNIT VACCINES
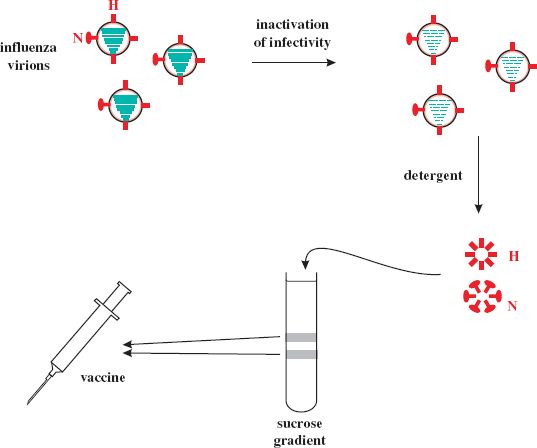
A subunit vaccine contains purified components of virions. In the case of influenza the vaccines contain the hemagglutinin (H) and neuraminidase (N) surface glycoproteins (Section 16.2). A typical production method is outlined in Figure 25.3. The infectivity of a batch of influenza virions is inactivated with formaldehyde or β-propiolactone, then the virion envelopes are removed with a detergent, such as Triton X-100. This releases the glycoproteins, which form aggregates of H “cartwheels” and N “rosettes.” These structures are purified by centrifugation in a sucrose gradient, and then material from three influenza virus strains is combined to form the vaccine.
Figure 25.3 Outline of production method for influenza virus subunit vaccine. Hemagglutinin (H) and neuraminidase (N) are extracted from inactivated influenza virions and purified by sucrose gradient centrifugation. The bands from the gradient are harvested and incorporated into the vaccine.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


