Chapter 14 After studying this chapter, the learner will be able to: • Differentiate between microorganisms. • Describe the natural lines of defense of the human body. • Identify several favorable living characteristics of microorganisms. Microorganism that requires air or the presence of oxygen for maintenance of life. Microorganism that grows best in an oxygen-free environment or one that cannot tolerate oxygen (e.g., Clostridium species that causes gas gangrene). Substances, natural or synthetic, that inhibit growth of or destroy microorganisms. Used as therapeutic agents against infectious diseases; some are selective for a specific organism; some are broad-spectrum antibiotics. Chemical or pharmaceutical agent that destroys or inhibits growth of microorganisms. Three-dimensional layers of living bacteria embedded in a sticky matrix that persists on the surface of tissues and implanted medical devices. Commonly the cause of chronic infections, such as otitis media and rhinitis. Covert event involving introduction of microbial contamination and infection of humans or animals. Infectious disease process that developed or was incubating before the patient entered the health care facility. Transmission of microorganisms from patient to patient and from inanimate objects to patients and vice versa. Forms of bacterial classes clostridia and bacillus that are generated when living conditions are not favorable. Protective capsule that forms inside specific bacterial species encircles and protects the genetic matter to resist destructive forces, such as disinfection or sterilization. Study of occurrence and distribution of disease; the sum of all factors controlling the presence or absence of a disease. Bacteria and fungi normally inhabiting the body, resident or transient. Hospital-associated or acquired infection (HAI) Not present when the patient was admitted to the health care facility. Infection may occur at the surgical site or as a complication unrelated to the surgical site (formerly known as nosocomial infection). Invasion of the body by pathogenic microorganisms and the reaction of tissues to their presence and to toxins generated by the organisms. Living organisms, invisible to the naked eye, including bacteria, fungi, viruses, protozoa, yeasts, and molds. Microorganisms that do not normally invade tissue but are capable of causing infection or disease if introduced into the body mechanically through injury, such as tetanus bacillus, or when resistance of the host may be lowered, as by human immunodeficiency virus (HIV) infection. Opportunistic infection. Producing or capable of producing disease. Protein that contains no genetic material and in its pathologic form causes fatal neurodegenerative disease. Difficult to inactivate. Requires special handling. Severe toxic febrile state resulting from infection with pyogenic microorganisms, with or without associated septicemia. Septicemia is a clinical syndrome characterized by significant invasion into the bloodstream of microorganisms from a focus of infection in tissues. Microorganisms may multiply in the blood. Infection of bacterial origin carried through the bloodstream is sometimes referred to as bacteremia. Procedures followed to protect personnel from contact with blood and body fluids of all patients (formerly referred to as universal precautions). Secondary subsequent infection caused by a different microorganism that develops during or after antibiotic therapy. Bacteria pass through four phases during their colonization/duplication/reproductive stage: 1. Lag phase. The microorganism adjusts to the new environment. 2. Exponential (logarithmic, or log) growth phase. This is characterized by a maximum and constant rate of multiplication. If conditions are favorable, Escherichia coli (E. coli) can double in 20 minutes. Mycobacterium tuberculosis (tubercle bacillus) may require 5 hours. 3. Stationary phase. The multiplication rate and death of microbes equalize and balance. This period can last several days. 4. Death. The rate of microbial death exceeds the rate of multiplication. A few living cells can linger for several days or months but not at the same level as the growth phase. The rate of bacterial growth is depicted in Figure 14-1. The population of microbes will double during the log phase at a predictable interval referred to as generation time or doubling time. Changes in temperature, moisture, illumination, nutrients, and pH can influence the rate at which any microbe passes through doubling time. If all favorable living conditions are met, the microbe can proliferate into disease state. Bacteria secretes a slime that accumulates in layers known as biofilm. Any change in the favorable conditions can alter the amount of time it takes for the microbe to pass through any one of the four phases. Box 14-1 describes the favorable living conditions associated with the proliferation of microorganisms. The human body is remarkable in its ability to protect itself by intact barriers, membranes, and bacteriostatic secretions. Three lines of defense are particularly important for prevention of disease.1,4 • Skin. Stratified epithelium contains sweat (sudoriferous) and oil (sebaceous) glands, which are bactericidal. Natural florae inhibit each other. The epithelium must remain intact to afford protection. Desquamation and low pH impede bacterial colonization. • Mucous membranes. An effective barrier when intact, these line all natural body orifices except the ears, which secrete cerumen (ear wax). Mucous membranes have bactericidal properties and a slightly acidic pH. • Reflexes. Examples include vomiting, gagging, and blinking. • Sneeze. Mucociliary escalator of the respiratory tree moves mucus and debris from the respiratory tract. • Genitourinary/reproductive tracts. Immunoglobulin A (IgA), found in mucus, and enzymes are produced; these have a slightly acidic pH. Peristalsis is unidirectional. • Eyes. Enzymes and IgA protect the conjunctiva and structures of the eye. • Cellular level. Interferon is naturally formed in the cell to fight against viral attack. • Stomach acid. Acid kills most pathogens. • Muscular closure of orifices. Muscular closure provides a mechanical barrier against orifices such as the cervix and sphincters. • Inflammatory response. This can be localized or systemic; biochemical, mechanical, and anatomic activities fight invading microorganisms and lay the groundwork for healing. • Antibody production. Production is stimulated by the presence of an antigen. Some antibodies can be replicated for future exposures to the same microorganism. • Temperature elevation. This can be localized or systemic. Some microorganisms are destroyed or repelled by heat. • Passive immunity. A “preformed” immunoglobulin (antibody) is introduced into the body. No memory for replication of the protective antibodies remains in the body. • Active immunity. The body has the ability to develop a memory for production of antibodies in response to specific antigens. Live, dead, or attenuated microorganisms trigger the response. Some exposures require a booster injection to spark the memory and maintain immunity. Veins are particularly vulnerable, because they are often associated with venous sinuses and have low pressure. Venous stasis permits the growth of microbial colonies. The central nervous system (CNS) shares a similar risk for infection because the cerebrospinal fluid is under lowered pressure and has components that are rich in nutrients. As microbial colonies increase in number, infection may be carried through the body in the lymphatic system and may eventually become bloodborne. Major organ systems can become involved and can result in multisystem organ failure and death. Any body fluid or substance is a potential carrier of pathogens (Box 14-2). According to the CDC, 14% to 16% of all health care–associated infections are surgical-site infections (SSI). Approximately 77% of surgical patients who die are reported to die of sepsis associated with these infections. SSI increases the length of stay and increases the cost of care.5 Biofilm forms when one or more species of bacteria, fungi, and other microorganisms adhere in layers to moistened surfaces, such as biologic tissue, implantable metals, and plastics (Box 14-3). The slimy matrix that binds the microorganisms together creates a barrier against antibiotic treatment that results in a persistent disease state. Plaques can break off and attach elsewhere, causing a separate biofilm colony. Biofilm can form on any biologic tissue surface or on inert devices implanted in the body, such as catheters, artificial joints, and mechanical heart valves. The biofilm begins as a single cell layer that attaches to a surface and multiplies and thickens rapidly (Fig. 14-2). Research has shown that biofilm is a genetically mediated process in which bacteria exchange intercellular information that give rise to newly formed biofilm in a process known as quorum sensing. A biofilm infection may linger for months, years, or even a lifetime, regardless of the state of the patient’s intact immune system. Bacteria in biofilm can be difficult to eliminate. The National Institutes of Health (NIH) points out that 75% of human infections are attributed to biofilm.7 An infected implant may need to be explanted. Infections may be caused by one or a combination of microorganisms. Each type of microorganism has a specific set of characteristics that promotes survival and proliferation. Knowing the specific needs for microbial life aids in the prevention of infection. In this chapter, each of the five main types of microorganisms is described according to structure, life cycle, and mode of transmission. Examples of each type of microorganism are provided in Table 14-1. TABLE 14-1 Common Microorganisms in an OR Environment More than 5000 species of bacteria have been named, and many more exist unidentified. Most of the morphologic differences in bacteria are found in metabolism, chemical composition, or resultant effect on the host. Unfortunately, many varieties of bacteria are pathogenic or are capable of becoming pathogenic (Fig. 14-3). 1. Structure. Bacteria are microscopic, single-cell structures (1 to 10 mm). Two billion bacteria can be contained in a single drop of water. Bacteria have DNA but no formal nucleus (prokaryotic) and no membrane-bound organelles. a. Cocci are round. Examples include strains of Staphylococcus and Streptococcus. Diseases include impetigo and gonorrhea. b. Bacilli are rod shaped, and some can form endospores. They are the most common types of bacteria. Examples include Bacillus, Clostridium, Escherichia, Proteus, and Pseudomonas species. c. Spirochetes are spiral shaped. Diseases include syphilis, leptospirosis, and Lyme disease. Syphilis and Lyme disease can have fatal neurologic effects in the later stages of illness known as neurosyphilis and neuroborreliosis (Lyme).3 d. Pleomorphs can change shape from rod to round, making positive identification difficult. Diseases include mycoplasmal infection, typhus, rickettsial infection, chlamydia, psittacosis, and Rocky Mountain spotted fever. Rickettsia and chlamydia organisms must live in a host cell. They are intracellular parasites. 2. Life cycle. Bacteria can be aerobic, anaerobic, facultative, or microaerophilic. They can reproduce asexually by binary fission (split into equal halves). Some studies have shown that some genes may be transferred between bacterial species during viral infection (plasmid transfer). 3. Communication. Some bacteria are capable of communicating that conditions are adequate for reproduction and colonization. The process of cells gathering and communicating is known as quorum sensing.7 When the cells have populated an area to a sufficient degree, the process of formal infection takes place. Quorum sensing is commonly found in biofilm accumulations. Studies have shown that engineered nanofactories show promise in the disruption of this form of communication between cells, thus decreasing the need for antibiotic administration. This works by allowing the bacteria to think there are enough cells in the area to cause infection. In reality, the bacterial presence triggers immune response and there are not enough bacterial cells to combat the body’s white cell activity. This may prove useful when antibiotic resistance is present because antibiotics do not play a role. 4. Transmission. Infection is passed along by direct contact or through animal or insect bites. 5. Encapsulation. Some bacteria are enclosed within a thick wall, which is a defense mechanism against phagocytic activity of leukocytes. These bacteria may be ingested by white blood cells, but instead of being killed and digested, they remain within the phagocyte for a time and are then extruded in a viable condition. The presence of a capsule is associated with virulence among pathogenic bacteria. This is not to be confused with endospore formation, which is discussed in later paragraphs. A universal way to determine the differences in bacteria is to stain the cell. Danish physician Christian Gram (1853-1938) developed a method of applying a solution of crystal violet and iodine (gentian violet) to the cell wall (bacterial coat) followed by exposure to 95% alcohol and acetone. Gram-positive bacteria retain a stain of dark purple-blue. Gram-negative bacteria retain only a stain of light pink after the rinsing process (Table 14-2). Gram stain is also used to identify nonbacterial substances such as trophocysts (helminth eggs) and larvae. TABLE 14-2
Surgical microbiology and antimicrobial therapy
Microorganisms: nonpathogens versus pathogens
Identification of microorganisms
Viability of microorganisms
Three lines of defense
Pathogenic invasion
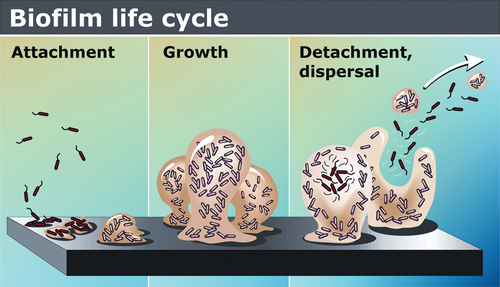
Biofilm
Types of pathogenic microorganisms
Microorganism
Usual Environment
Mode of Transmission
Staphylococci
Skin, hair
Direct contact
Upper respiratory tract
Airborne
Escherichia coli
Intestinal tract
Feces, urine
Urinary tract
Direct contact
Streptococci
Oronasopharynx
Airborne
Skin, perianal area
Direct contact
Mycobacterium tuberculosis
Respiratory tract
Airborne, droplet
Urinary tract
Direct contact
Pseudomonas
Urinary tract
Direct contact
Intestinal tract
Urine, feces
Water
Water
Serratia marcescens
Urinary tract
Direct contact
Respiratory tract
Water
Clostridium
Intestinal tract
Direct contact
Fungi
Dust, soil
Airborne
Inanimate objects
Direct contact
Hepatitis virus
Blood
Bloodborne
Body fluids
Direct contact
Bacteria
Characteristics
Differentiation of bacterial types
Gram stain.
Gram-positive Cocci
Gram-negative Cocci
Gram-positive Rods
Gram-negative Rods
Aerobic
Staphylococcus aureus
Neisseria gonorrhoeae
Listeria monocytogenes
Escherichia coli
Staphylococcus epidermidis
Neisseria meningitidis
Bacillus anthracis
Klebsiella pneumoniae
Streptococcus pneumonia
Moraxella catarrhalis
Corynebacterium diphtheriae
Proteus mirabilis
Streptococcus pyogenes
Serratia marcescens
Streptococcus viridans
Pseudomonas aeruginosa
Enterococcus faecalis
Enterobacter
Enterococcus faecium
Haemophilus influenzae
Legionella pneumophila
Salmonella
Shigella
Brucella
Bortadella
Campylobacter
Anaerobic
Peptostreptococcus
Clostridium difficile
Bacteroides fragilis
Peptococcus
Clostridium perfringens
Fusobacterium
Clostridium tetani
Actinomyces
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



 Website
Website