 Superior Thoracic Aperture and Cervicothoracic Sympathetic Chain
Superior Thoracic Aperture and Cervicothoracic Sympathetic ChainAnatomy of the “Thoracic Outlet”
The superior opening of the bony thorax has come to be called the thoracic outlet. The anatomic term superior thoracic aperture and the term thoracic outlet will be used interchangeably in this chapter to designate the regional anatomy.
Compression of upper extremity neurovascular structures, collectively called the thoracic outlet syndrome, encompasses considerably more anatomy than the cephalad aperture of the bony thorax. The vessels exiting the chest and the nerves emerging from the spinal column pass between the scalene muscles above the rim of the superior thoracic aperture. They then pass through the triangle formed by the first rib, clavicle, and scapula and run beneath the coracoid process on their way to the brachium.
The following discussion considers all of the structures that can compress and compromise the nerves and blood vessels of the upper extremity. The basic surgical approaches to correcting such compression are addressed in the second part of the chapter.
The Cephalad Passages
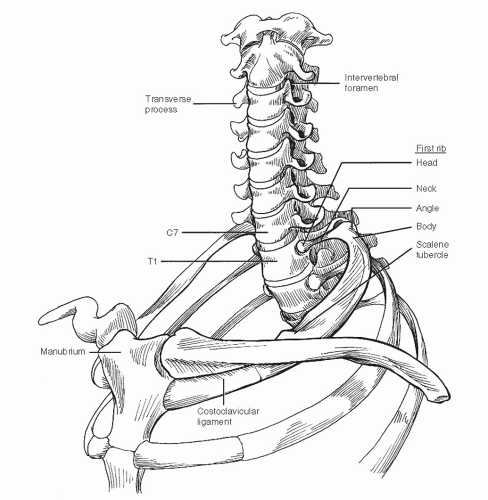
The superior thoracic aperture is bounded by the first ribs, which connect the spinal column posteriorly with the sternum anteriorly (Fig. 4-1). The vertebral bodies indent the oval shape of this opening. The manubrium of the sternum rises above the plane of the first ribs to articulate with the heads of the clavicles. The mobile sternoclavicular joint is the only osseous connection between the axillary skeleton and the bones of the upper extremity. The mobility of the clavicle is important in determining the amount of space available for passage of the subclavian vessels and brachial plexus draped over the first rib. The costoclavicular ligament as well as the sternoclavicular joint attach the clavicle medially.
The transverse processes of the cervical vertebrae are trough-shaped and contain central apertures. The vertebral arteries normally enter the sixth transverse foramen and traverse the upper five foramina to reach the base of the skull. The transverse process of the seventh cervical vertebra is often quite large. Rarely, a cervical rib may be present, which attaches to this transverse process and lies in the path of the brachial plexus.
The fifth through the eighth cervical nerves and the first thoracic nerve give origin to the brachial plexus (Fig. 4-2). The nerves emerge through the intervertebral foramina and lie in the troughs of the transverse processes posterior to the vertebral vessels. The anterior and middle scalene muscles sandwich the roots of the brachial plexus. The anterior scalene muscles originate from the anterior tubercles of transverse processes three through six and insert on the scalene tubercle of the first rib between the subclavian artery and vein. The middle scalene muscles arise from the posterior tubercles of the lower six cervical transverse processes and attach more broadly to the posterior parts of the first ribs. The posterior scalene muscle rarely contributes to the thoracic outlet syndrome.
The Neurovascular Core
The roots of the brachial plexus are the ventral rami of the fifth through eighth cervical and first thoracic nerves, which lie between the anterior and middle scalene muscles (Fig. 4-3). Topographically these lie in the posterior triangle of the neck. As they emerge between the scalene muscles, the roots of the brachial plexus unite to form three trunks. At the level of the first rib, the trunks divide into anterior and posterior divisions which lie posterior to the first part of the axillary artery. The posterior divisions unite to form the posterior cord which continues behind the axillary artery to become the radial nerve. The anterior divisions form lateral and medial cords around the artery. The medial cord gives rise to the ulnar nerve. A branch of the medial cord unites with the lateral cord to form the median nerve anterior to the artery.
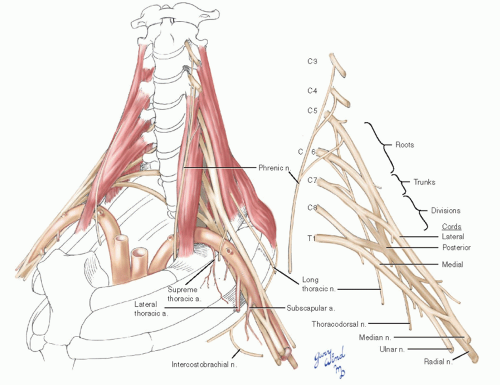 Fig. 4-3 The relationship between the brachial plexus and subclavian/axillary arteries is depicted in this illustration. |
There are three important branches which deviate from the central location of the brachial plexus. Twigs from the roots of the fifth, sixth, and seventh nerves unite to form the long thoracic nerve and pass through the substance of the middle scalene muscle to reach the serratus anterior muscle on the chest wall. This relationship is important during the posterior dissection for first rib resection.
The phrenic nerve arises from the third, fourth, and fifth ventral nerve roots. It descends on the surface of the anterior scalene muscle, running from a lateral to a medial position. It enters the chest
between the subclavian artery and vein at the inner margin of the first rib, just medial to the anterior scalene attachment to the scalene tubercle.
between the subclavian artery and vein at the inner margin of the first rib, just medial to the anterior scalene attachment to the scalene tubercle.
Another branch of the brachial plexus that is notable by its proximity during surgical correction of thoracic outlet compression syndromes is the thoracodorsal nerve. It arises from the posterior cord in the midaxilla and joins the thoracodorsal vessels to the latissimus dorsi muscle. Its lateral origin puts it at the periphery of the operative field when the arm is elevated.
The intercostobrachial nerve is a branch of the second intercostal nerve which crosses the center of the axillary space and usually joins the medial brachial cutaneous nerve. Some numbness on the inner aspect of the brachium may result from division of this nerve.
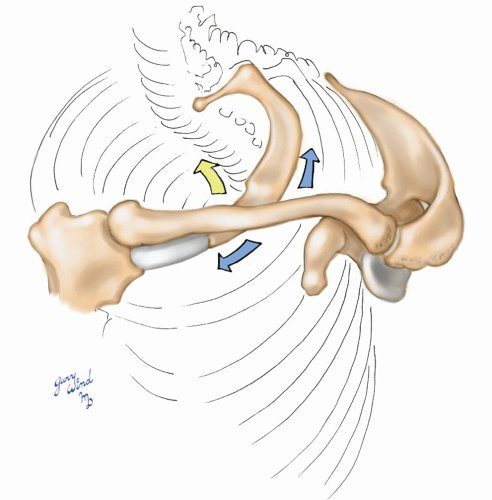 Fig. 4-4 The great mobility of the pectoral girdle affects the amount of space available for the nerve and vascular trunks to the upper extremity. |
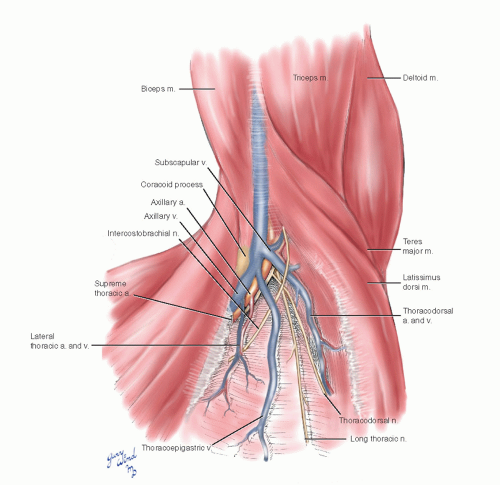
The axillary artery gives off the small supreme thoracic artery at the lateral margin of the first rib, the lateral thoracic artery in the midaxilla, and the subscapular artery in the distal axilla. The supreme and lateral thoracic vessels lie directly on the chest wall. The subscapular vessels arise more laterally and are not directly on the chest wall.
The Axillary Passages
The mobile pectoral girdle frames the upper border of the axilla (Fig. 4-4). The muscles attaching the clavicle and scapula to the chest wall form the boundaries of the axilla and create the neurovascular bundle’s passageway to the upper extremity. Elevation of the pectoral girdle widens the passage, while depression and posterior displacement narrow the space.
The serratus anterior muscle forms the majority of the medial axillary wall (Fig. 4-5). The subclavius muscle forms a bridge from the undersurface of the distal clavicle to the costochondral junction of the first rib. A second bridge is formed by the arching coracoid process and the origin of the pectoralis minor muscle. Subscapularis, teres major, and latissimus dorsi muscles form the posterolateral boundary of the axilla. The neurovascular bundle passes under these bridges to reach the axillary space.
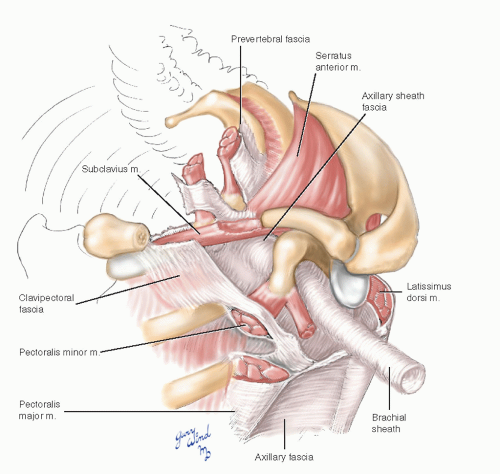 Fig. 4-5 The clavipectoral and axillary fasciae form anterior and lateral walls of the axillary space. |
The axillary space and neurovascular bundle are enclosed by several well-defined fascial layers. The prevertebral fascia surrounding the scalene muscles continues onto the surface of the vessels and nerves lying over the first rib, forming an axillary sheath. The clavipectoral fascia extends from the subclavius muscle across the pectoralis minor muscle, which it enfolds, and joins the axillary fascia. The latter spans from the lateral edge of the pectoralis major muscle to the anterior edge of the latissimus dorsi.
Looking from below the clavicle, one can see the geometry which causes compression of the costoclavicular passage with depression and posterior movement of the clavicle (Fig. 4-6). The angle between the clavicle and first rib is acute and subject to scissors-like closure, with the most pronounced effect being on the subclavian vein lying between them. The insertion of the subclavius muscle forms the medial margin of the foramen through which the subclavian vein enters the chest. It is an important landmark toward which the needle is directed when cannulating the subclavian vein, and it is the highest point of a complete axillary lymph node dissection.
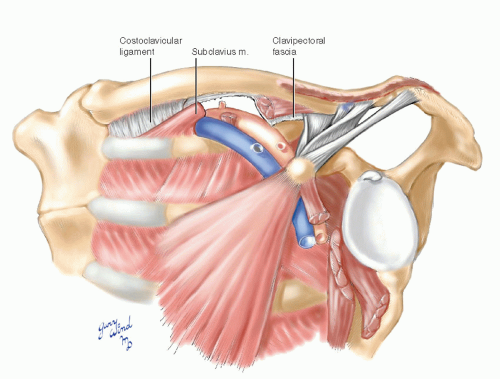 Fig. 4-6 Oblique fibers of the pectoralis minor and major and the latissimus dorsi make the distal clavicle a lever that closes the costoclavicular angle. |
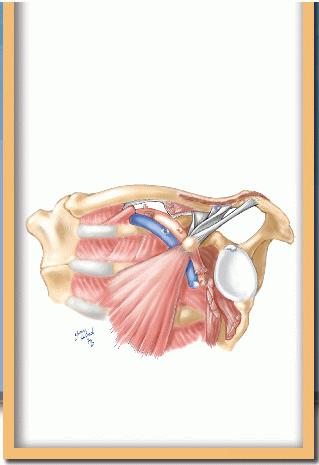
The surgical view of the axilla shows the relationships of the neurovascular bundle, its branches, and the surrounding structures (Fig. 4-7). The supreme thoracic and lateral thoracic vessels and thoracoepigastric vein cross the critical portion of the first rib and must be divided for access. The long thoracic and lateral thoracic nerves posterolaterally are important to preserve. The intercostobrachial nerve may be preserved or divided, depending on whether it interferes with exposure.
Vasomotor Sympathetics
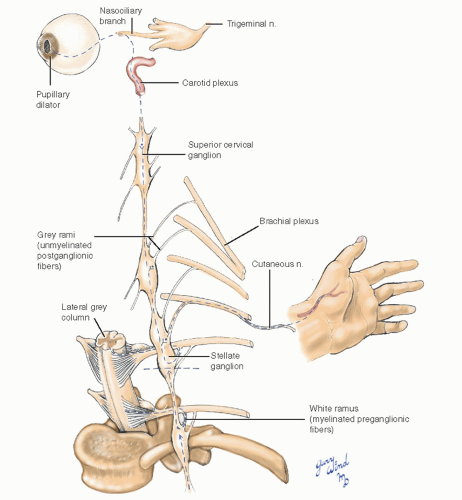
Autonomic control of vascular tone in the upper extremity is regulated by sympathetic nerves whose preganglionic fibers originate in the lateral grey column of the second to eighth thoracic spinal cord segments. The axons pass from the cord through the ventral nerve root to the ventral rami where they exit via the white rami to the sympathetic trunk (Fig. 4-8). Within the sympathetic trunk, the preganglionic fibers synapse with multiple postganglionic neurons at various levels. Postganglionic axons from the middle cervical, stellate, and second thoracic ganglia join the roots of the brachial plexus or run directly in the adventitia of blood vessels. The majority of upper extremity vascular sympathetic nerves reach their destination through the lower trunk of the brachial plexus and the median and ulnar nerves.
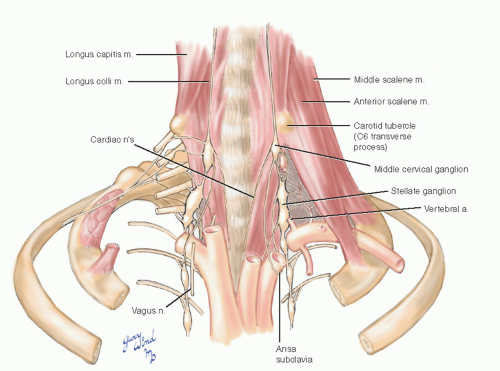
Anatomically, there are fewer thoracolumbar sympathetic ganglia than there are cord segments. The stellate ganglion found in most individuals represents the fusion of the inferior cervical and the first thoracic ganglia. The cervical sympathetic chain on each side lies between the carotid sheath and the prevertebral fascia anterior to the transverse processes of the cervical vertebrae (Fig. 4-9). The middle cervical ganglion lies at the level of the C6 transverse process (carotid tubercle) medial to the vertebral artery as it enters the vertebral foramen. The trunk then turns posterolateral to assume a paravertebral position. The thin ansa subclavia loops around the subclavian artery between the middle cervical ganglion and the stellate ganglion. In the chest, the sympathetic trunks and ganglia lie beneath the parietal pleura on the necks of the ribs.
Complete interruption of the sympathetic innervation of the upper extremity also interrupts the innervation of the pupillary dilator, local facial sweat glands, and blood vessels, resulting in Horner’s syndrome. Section of the sympathetic chain at the lowermost part of the stellate ganglion preserves enough TI sympathetic outflow in most cases to avoid a Horner’s syndrome. The amount of sympathetic innervation left in the upper extremity is not felt to be clinically significant by advocates of this procedure.
An older controversy involved the denervation hypersensitivity to circulating catecholamines, which results from interruption of postganglionic sympathetic fibers. Older procedures to selectively divide white rami only have been abandoned, since this syndrome is rarely observed clinically.
Exposure of the Thoracic Outlet
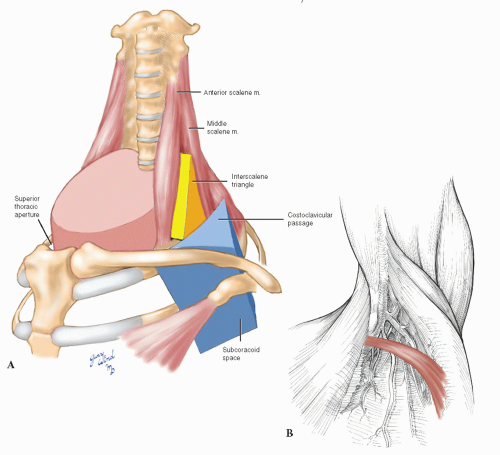
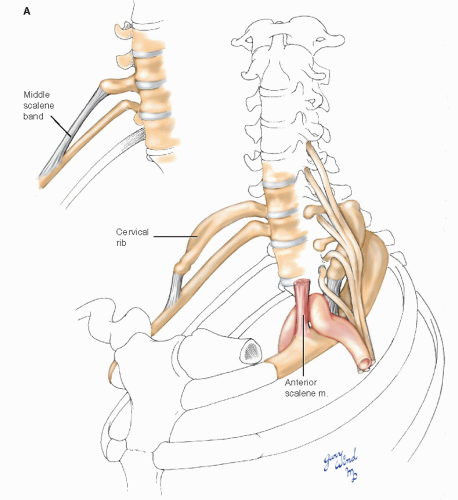
Compression syndromes involving neurovascular structures in the thoracic outlet have been recognized for many years. The anatomic situation underlying compression in this area is the normal existence of four narrow spaces through which the neurovascular bundle must pass in coursing from the neck to the axilla: the superior thoracic aperture, interscalene triangle, costoclavicular passage, and subcoracoid space. Thyromegaly, thymic lesions, or adenopathy may reduce space availability within the superior thoracic aperture1 (Fig. 4-10A). The interscalene triangle is a narrow confine bordered by the anterior scalene muscle anteriorly, the middle scalene muscle posteriorly, and the first rib inferiorly. The presence of first rib anomalies, fibromuscular bands, or abnormal muscular insertions can result in a fibromuscular vise that compresses the subclavian vessels and brachial plexus in this area.2,3,4 The costoclavicular passage is a second triangle made up of the subclavius muscle and clavicle anteriorly, the first rib posteromedially, and the scapula and subscapularis muscle posterolaterally. This area is a common site of subclavian vein compression in patients with effort thrombosis.5,6 A third anatomic triangle exists in the subcoracoid space, where the neurovascular bundle passes between the coracoid process and the pectoralis minor tendon. A tight band of superior clavipectoral fascia, the costocoracoid ligament, may narrow the subcoracoid space during shoulder abduction. Hypertrophy of the pectoralis minor muscle in athletes may also cause subcoracoid space narrowing.7 More laterally, an accessory muscle that crosses the axilla from the latissimus dorsi to the pectoralis major muscle, Langer’s axillary arch (Fig. 4-10B), has been reported to cause upper limb deep vein thrombosis from compression.8
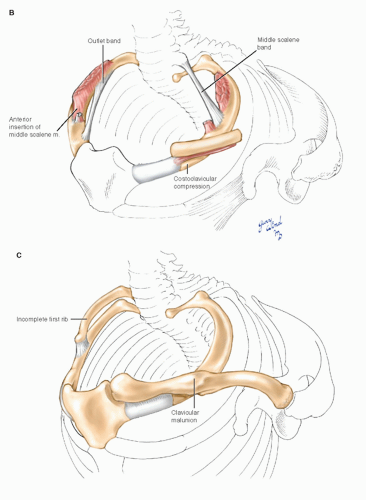
Space availability within the confines of these narrow apertures may be further reduced by a combination of physiologic, anthropometric, and pathologic factors (Fig. 4-11). Hypertrophy of the anterior scalene or the pectoral muscles, scoliosis, abnormal shoulder posture, existence of cervical ribs, and clavicular fracture malunions, and other shoulder injuries have all been implicated in compression syndromes.2,3,9 In addition, the existence of abnormal myofascial bands or anomalous scalene muscle insertions may underlie compression symptoms. Roos11 feels that congenital fibrous bands or abnormal muscle insertions are the most common causes of this disorder. However, since these anomalies are common in the general population,4,9,12 others have suggested that local trauma may be an important causative influence in patients who are predisposed to compression syndromes by congenital anomalies.2,9
There are three discrete types of thoracic outlet compression syndromes: brachial plexus compression, subclavian artery compression, and subclavian vein compression. Neural compression is by far the most common type, accounting for nearly 97% of thoracic outlet compression symptoms.2,3,13 Diagnosis and evaluation of patients with all three types of suspected thoracic outlet compression are well-described elsewhere.2,3,14 It should be stressed that a majority of patients will experience relief of neurologic symptoms with nonoperative therapy.2,3 In a recent review, Brooke and Freischlag13 noted that injecting lidocaine or botulinum toxin A (botox) into the anterior scalene muscle at its insertion onto the first rib may provide durable symptom relief of neurogenic thoracic outlet syndrome for up to 3 months. Moreover, the degree of symptom relief appears to correlate with successful response to physical therapy and surgical intervention.
Several surgical procedures have been described for the treatment of thoracic outlet compression syndromes. Early reports suggested that simple scalenectomy was effective in alleviating symptoms.15 Although some authors suggest that anterior scalene muscle excision may be appropriate in highly selected patients,2,16 the consensus of opinion is that the first rib should be removed because it is the anatomic structure common to all three of the narrow thoracic apertures described above (Fig. 4-12). Clagett17 was the first to recommend routine resection of the first rib as a way to alleviate symptoms regardless of the site of compression. Most modern surgeons have adopted this view and remove the first rib through one of two approaches, transaxillary and supraclavicular.
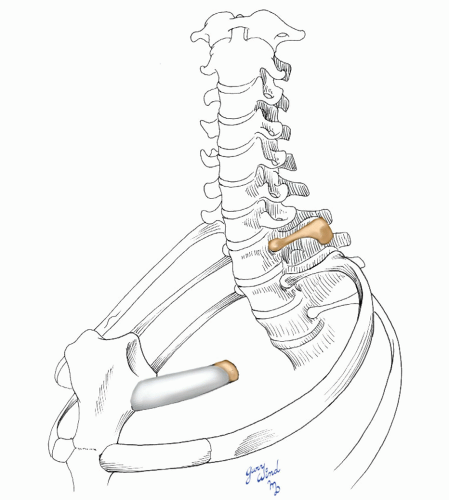 Fig. 4-12 The compression from a variety of causes responds to removal of the body of the first rib. |
The transaxillary approach has been favored for many years. It is cosmetically superior but difficult to perform in muscular individuals. The anterior supraclavicular approach has undergone rejuvenation and is currently favored by some.18,19,20 This approach should be used in cases involving agenesis of the first rib and when arterial reconstruction is planned. The posterior approach originally described by Clagett17 has been abandoned because it
is technically demanding. The anterior infraclavicular approach21 offers only limited first rib resection, and the view of the neurovascular structures is obstructed by the clavicle. It is most useful to ensure sternal disarticulation of the first rib and complete resection of the subclavius muscle in patients with venous thoracic outlet compression (see below). The transclavicular approach requires resection of the clavicle and is most useful in cases involving clavicular pathology. Because of the high reported risk of postoperative shoulder pain, claviculectomy is not recommended for thoracic outlet decompression in most cases.22 The following discussion concerns the three currently popular approaches to thoracic outlet decompression.
is technically demanding. The anterior infraclavicular approach21 offers only limited first rib resection, and the view of the neurovascular structures is obstructed by the clavicle. It is most useful to ensure sternal disarticulation of the first rib and complete resection of the subclavius muscle in patients with venous thoracic outlet compression (see below). The transclavicular approach requires resection of the clavicle and is most useful in cases involving clavicular pathology. Because of the high reported risk of postoperative shoulder pain, claviculectomy is not recommended for thoracic outlet decompression in most cases.22 The following discussion concerns the three currently popular approaches to thoracic outlet decompression.
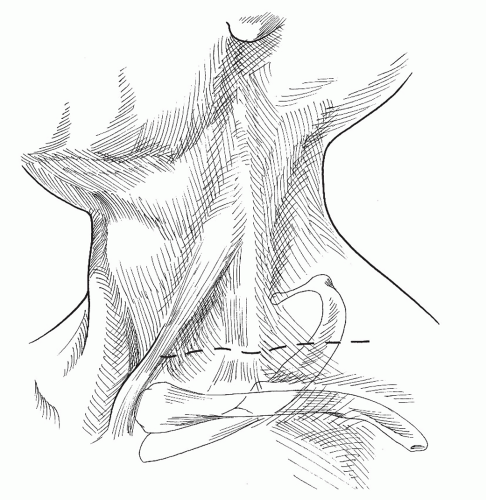
Anterior Supraclavicular Approach
The principal advantage of this approach is that it provides wide exposure of all anatomic structures of the thoracic outlet. It can also be combined easily with other incisions to correct arterial pathology at the time of outlet decompression. Although the supraclavicular approach has been used since 1910, it has become a popular method of thoracic outlet decompression within the last three decades.23




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree