Individual sulfonamides vary greatly with respect to solubility in water. Older sulfonamides had low solubility; therefore they often crystallized out in the urine, causing injury to the kidneys. The sulfonamides in current use are much more water soluble, and hence the risk for renal damage is low.
Mechanism of Action
Sulfonamides are usually bacteriostatic. Accordingly, adequate host defenses are essential for elimination of infection.
Sulfonamides suppress bacterial growth by inhibiting synthesis of tetrahydrofolate, a derivative of folic acid (folate). Folate is required by all cells to make DNA, RNA, and proteins. The steps in folate synthesis are shown in Fig. 73.2. Sulfonamides block the step in which PABA is combined with pteridine to form dihydropteroic acid. Because of their structural similarity to PABA, sulfonamides act as competitive inhibitors of this reaction.
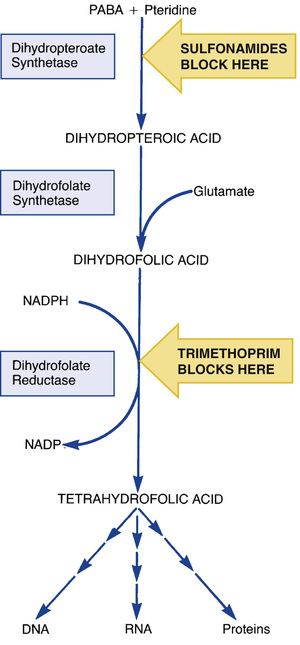
If all cells require folate, why don’t sulfonamides harm us? The answer lies in how bacteria and mammalian cells acquire folic acid. Bacteria are unable to take up folate from their environment, so they must synthesize folic acid from precursors. In contrast to bacteria, mammalian cells do not manufacture their own folate. Rather, they simply take up folic acid obtained from the diet, using a specialized transport system for uptake. Because mammalian cells use preformed folic acid rather than synthesizing it, sulfonamides are harmless to us.
Microbial Resistance
Many bacterial species have developed resistance to sulfonamides. Resistance is especially high among gonococci, meningococci, streptococci, and shigellae. Resistance may be acquired by spontaneous mutation or by transfer of plasmids that code for antibiotic resistance (R factors). Principal resistance mechanisms are (1) reduced sulfonamide uptake, (2) synthesis of PABA in amounts sufficient to overcome sulfonamide-mediated inhibition of dihydropteroate synthetase, and (3) alteration in the structure of dihydropteroate synthetase such that binding and inhibition by sulfonamides is reduced.
Antimicrobial Spectrum
The sulfonamides are active against a broad spectrum of microbes. Susceptible organisms include gram-positive cocci (including methicillin-resistant Staphylococcus aureus), gram-negative bacilli, Listeria monocytogenes, actinomycetes (e.g., Nocardia), chlamydiae (e.g., Chlamydia trachomatis), some protozoa (e.g., Toxoplasma species, plasmodia, Isospora belli), and two fungi: Pneumocystis jiroveci (formerly thought to be Pneumocystis carinii) and Paracoccidioides brasiliensis.
Therapeutic Uses
Although the sulfonamides were once employed widely, their applications are now limited. Two factors explain why: (1) introduction of bactericidal antibiotics that are less toxic than the sulfonamides and (2) development of sulfonamide resistance. Today, UTI is the principal indication for these drugs.
Urinary Tract Infections
Sulfonamides are often preferred drugs for acute UTIs. About 90% of these infections are due to Escherichia coli, a bacterium that is usually sulfonamide sensitive. Of the sulfonamides available, sulfamethoxazole (in combination with trimethoprim) is generally favored. Sulfamethoxazole has good solubility in urine and achieves effective concentrations within the urinary tract. UTIs are discussed in Chapter 74.
Other Uses
Sulfonamides are useful drugs for nocardiosis (infection with Nocardia asteroides), Listeria species infection, and infection with P. jiroveci. In addition, sulfonamides are alternatives to doxycycline and erythromycin for infections caused by C. trachomatis (trachoma, inclusion conjunctivitis, urethritis, lymphogranuloma venereum). Sulfonamides are used in conjunction with pyrimethamine to treat two protozoal infections: toxoplasmosis and malaria caused by chloroquine-resistant Plasmodium falciparum. Topical sulfonamides are used to treat superficial infections of the eyes and to suppress bacterial colonization in burn patients.
One sulfonamide—sulfasalazine—is used to treat ulcerative colitis. However, benefits in this disorder do not result from inhibiting microbial growth. Ulcerative colitis is discussed in Chapter 64.
Pharmacokinetics
Absorption
Sulfonamides are well absorbed after oral administration. When applied topically to the skin or mucous membranes, these drugs may be absorbed in amounts sufficient to cause systemic effects.
Distribution
Sulfonamides are well distributed to all tissues. Concentrations in pleural, peritoneal, ocular, and similar body fluids may be as much as 80% of the concentration in blood. Sulfonamides readily cross the placenta, and levels achieved in the fetus are sufficient to produce antimicrobial effects and toxicity.
Metabolism
Sulfonamides are metabolized in the liver, principally by acetylation. Acetylated derivatives lack antimicrobial activity but are just as toxic as the parent compounds. Acetylation may decrease sulfonamide solubility, thereby increasing the risk for renal damage from crystal formation.
Excretion
Sulfonamides are excreted primarily by the kidneys. Thus the rate of renal excretion is the principal determinant of their half-lives.
Adverse Effects
Sulfonamides can cause multiple adverse effects. Prominent among these are hypersensitivity reactions, blood dyscrasias, and kernicterus, which occurs in newborns. Renal damage from crystalluria was a problem with older sulfonamides but is less common with the sulfonamides used today.
Hypersensitivity Reactions
Sulfonamides can induce a variety of hypersensitivity reactions, which are seen in about 3% of patients. Mild reactions—rash, drug fever, photosensitivity—are relatively common. To minimize photosensitivity reactions, patients should avoid prolonged exposure to sunlight, wear protective clothing, and apply a sunscreen to exposed skin.
Hypersensitivity reactions are especially frequent with topical sulfonamides. As a result, these preparations are no longer employed routinely. Rather, they are usually reserved for ophthalmic infections, burns, and bacterial vaginosis caused by Gardnerella vaginalis and a mixed population of anaerobic bacteria.
The most severe hypersensitivity response to sulfonamides is Stevens-Johnson syndrome, a rare reaction with a mortality rate of about 25%. Symptoms include widespread lesions of the skin and mucous membranes, combined with fever, malaise, and toxemia. The reaction is most likely to occur with long-acting sulfonamides, which are now banned in the United States. Short-acting sulfonamides may also induce the syndrome, but the incidence is low. To minimize the risk for severe reactions, sulfonamides should be discontinued immediately if skin rash of any sort is observed. In addition, sulfonamides should not be given to patients with a history of hypersensitivity to chemically related drugs, including thiazide diuretics, loop diuretics, and sulfonylurea-type oral hypoglycemics—although the risk for cross-reactivity with these agents is probably low (see later section “Drug Interactions”).
Hematologic Effects
Sulfonamides can cause hemolytic anemia in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency. This inherited trait is most common among blacks and people of Mediterranean origin. Rarely, hemolysis occurs in the absence of G6PD deficiency. Red cell lysis can produce fever, pallor, and jaundice; patients should be observed for these signs. In addition to hemolytic anemia, sulfonamides can cause agranulocytosis, leukopenia, thrombocytopenia, and, very rarely, aplastic anemia. When sulfonamides are used for a long time, periodic blood tests should be obtained.
Kernicterus
Kernicterus is a disorder in newborns caused by deposition of bilirubin in the brain. Bilirubin is neurotoxic and can cause severe neurologic deficits and even death. Under normal conditions, infants are not vulnerable to kernicterus. Any bilirubin present in their blood is tightly bound to plasma proteins and therefore is not free to enter the central nervous system (CNS). Sulfonamides promote kernicterus by displacing bilirubin from plasma proteins. Because the blood-brain barrier of infants is poorly developed, the newly freed bilirubin has easy access to sites within the brain. Because of the risk for kernicterus, sulfonamides should not be administered to infants younger than 2 months. In addition, sulfonamides should not be given to pregnant patients after 32 weeks of gestation or to those who are breastfeeding.
PATIENT-CENTERED CARE ACROSS THE LIFE SPAN
Sulfonamides and Trimethoprim
| Life Stage | Patient Care Concerns |
| Infants | Use of sulfonamides in infants younger than 2 months can cause kernicterus, a potentially fatal condition. |
| Pregnant women | Systemic sulfonamides are classified in U.S. Food and Drug Administration Pregnancy Risk Category D. They may cause birth defects, especially if taken during the first semester. If taken near term, the infant may develop kernicterus. |
| Breastfeeding women | Sulfonamides are secreted in breast milk. Breastfeeding women should be warned that breastfeeding an infant younger than 2 months can cause kernicterus. |
| Older adults | Older patients are more likely to experience adverse effects, and, when experienced, the effects are more likely to be severe. Life-threatening effects, including neutropenia, Stevens-Johnsons syndrome, and toxic epidermal necrolysis, occur more frequently in older adults. |
Renal Damage From Crystalluria
Because of their low solubility, older sulfonamides tended to come out of solution in the urine, forming crystalline aggregates in the kidneys, ureters, and bladder. These aggregates cause irritation and obstruction, sometimes resulting in anuria and even death. Renal damage is uncommon with today’s sulfonamides, owing to their increased water solubility. To minimize the risk for renal damage, adults should maintain a daily urine output of at least 1200 mL. This can be accomplished by consuming 8 to 10 glasses of water each day. Because the solubility of sulfonamides is highest at elevated pH, alkalinization of the urine (e.g., with sodium bicarbonate) can further decrease the chances of crystalluria.
Drug Interactions
Metabolism-Related Interactions
Sulfonamides can intensify the effects of warfarin, phenytoin, and sulfonylurea-type oral hypoglycemics (e.g., glipizide, glyburide). The principal mechanism is inhibition of hepatic metabolism. When combined with sulfonamides, these drugs may require a reduction in dosage to prevent toxicity.
Cross-Hypersensitivity
There is concern that people who are hypersensitive to sulfonamide antibiotics may be cross-hypersensitive to other drugs that contain a sulfonamide moiety (e.g., thiazide diuretics, loop diuretics, sulfonylurea-type oral hypoglycemics). However, there are no good data to show that such cross-hypersensitivity actually exists. In fact, clinical experience has shown that patients with documented allergy to sulfonamide antibiotics have taken other sulfonamide drugs without incident. Still, until more is known regarding cross-hypersensitivity, it is best to avoid taking chances unless the benefits of giving a drug are greater than the risks.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


