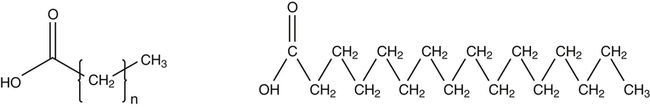
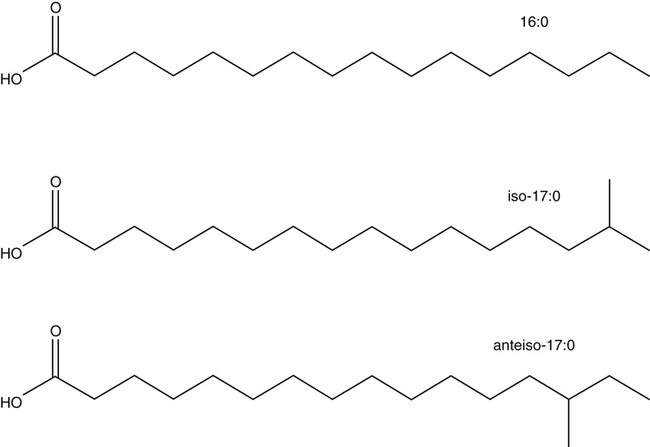
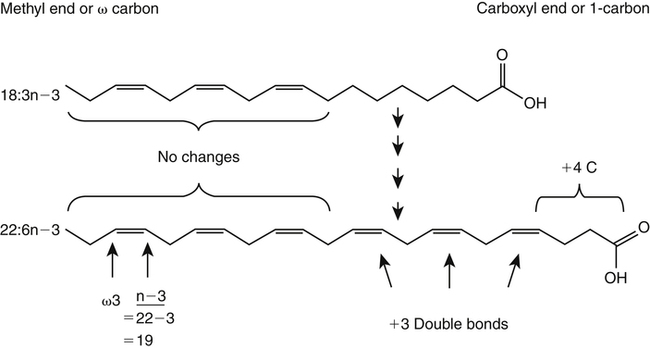
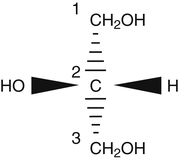
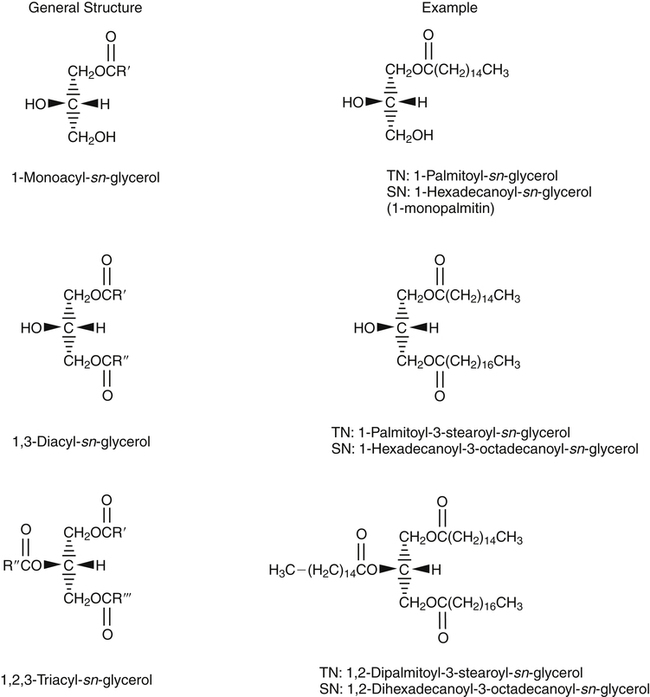
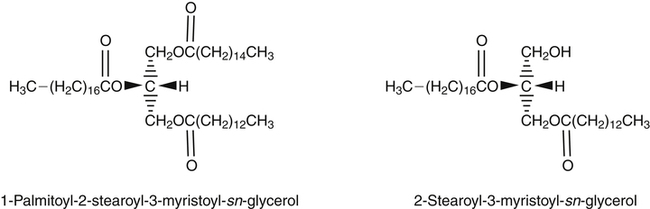
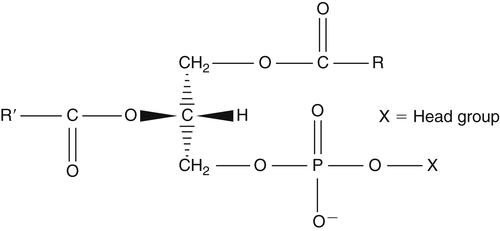
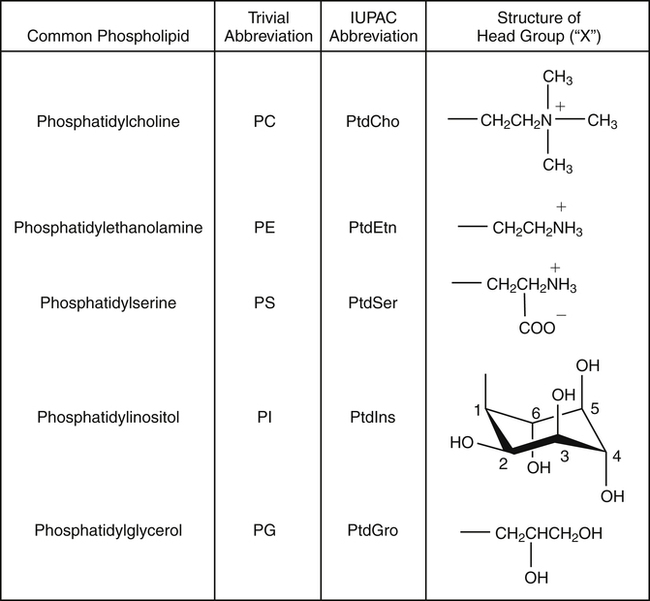
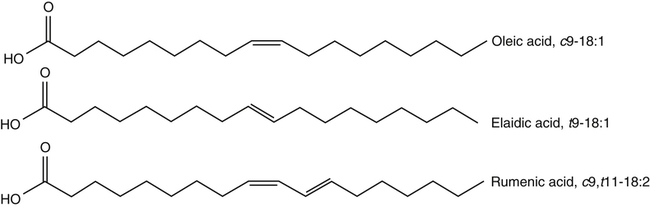
J. Thomas Brenna, PhD and Gavin L. Sacks, PhD Lipids do not share any overall characteristic chemical structural similarity, but they can nevertheless be categorized into subclasses according to structural similarities. One system for categorizing major classes of lipids is listed in Box 6-1, emphasizing those that are directly and indirectly of nutritional importance. A comprehensive system oriented toward detailed molecular studies is available at Lipidomics Gateway (www.lipidmaps.org/). The nomenclature of lipids is dominated by trivial names that are either historical or driven by systematics of metabolism. As with all organic compounds, systematic organic chemical naming rules have been established for lipids, but traditional lipid nomenclature persists for good reason. Most traditional lipid naming conventions are convenient when viewed in the context of mammalian lipid metabolism and, to a lesser extent, are logical extensions of the traditional methods used to analyze lipids. Thus study of lipid nomenclature also reveals structural and metabolic relationships among lipids, and familiarity with nomenclature makes the study of lipid metabolism much clearer. The term saturated in the context of fatty acids refers specifically to fatty acyl chains that are exclusively made up of sp3 hybridized (tetrahedral geometry) carbon atoms arranged as linear −CH2− chains as shown in Figure 6-1. Such chains are said to be saturated with hydrogen because the chain has no double (or triple) bonds across which hydrogen may be added. Fatty acids are synthesized predominantly through the successive addition of two-carbon units to a growing acyl chain by the fatty acid synthase enzyme. Chain-elongation ceases at 16 carbons to create palmitic acid in mammals, although small amounts of fatty acids with 14- (myristic acid) and 18- (stearic acid) carbons are also produced. Additional two-carbon units may be added to these fatty acids by elongation enzyme systems to yield longer saturated fatty acids. Table 6-1 presents the chain lengths, systematic names, trivial names, and melting points of the most abundant saturated fatty acids in mammalian tissues. TABLE 6-1 Naturally Occurring Straight-Chain Saturated Carboxylic Acids and Their Melting Points MP, Melting point; n, normal, straight-chain structural isomer. In mammals, the majority of saturated fatty acids are configured as straight chains, but in rare cases methyl groups extend from the main chain, usually near its terminal methyl. A fatty acid that terminates with two methyl groups (i.e., an isopropyl configuration) at the end of a hydrocarbon chain is referred to as iso. If the chain terminates with a methyl and an ethyl group (i.e., an isobutyl configuration), the fatty acid is said to be anteiso, as shown in Figure 6-2. In humans, branched-chain fatty acids appear in vernix, the protective waxy white substance that coats and protects the fetus during late gestation. They have also been detected as minor components of skin, blood, and hair, as well as in cancer cells. Iso and anteiso C15 and C17 saturated fatty acids are major components of some microorganisms such as gram-positive bacteria. Unsaturated is used to describe fatty acids with at least one double bond, consisting of two adjacent sp2 hybridized carbon atoms, with a trigonal, approximately planar geometry. They are unsaturated with respect to hydrogen, because hydrogen can be covalently added across the double bond to yield sp3 carbon atoms. The overwhelming majority of unsaturated sites in mammalian fatty acids are double bonds configured in the cis geometry. When two or more double bonds are present in a molecule, the fatty acid is said to be polyunsaturated. Generally, double bonds in mammalian polyunsaturated fatty acids (PUFAs) are separated by a methylene (−CH2−) group (“methylene-interrupted”). This structural property of PUFAs confers special chemical properties. One important chemical property is that the double bonds are not conjugated, and thus there is free rotation about the −CH2− group. As with saturated fatty acids, use of trivial names is very common for unsaturated fatty acids. Table 6-2 is a compilation of the systematic and trivial names for the most common unsaturated fatty acids. TABLE 6-2 Naturally Occurring Straight-Chain Unsaturated Fatty Acids and Their Melting Points MP, Melting point. PUFA biosynthesis is discussed in depth in Chapter 18; however, a brief overview is necessary to rationalize PUFA nomenclature. Consider the pathway for synthesis of docosahexaenoic acid (C22, 6 double bonds) from α-linolenic acid (C18, 3 double bonds). This pathway begins with the insertion of a double bond between carbons 6 and 7 of α-linoleic acid by a Δ6-desaturase to make stearidonic acid (C18, 4 double bonds). Two carbons are then added to the carboxyl end of the molecule by an elongase, followed by insertion of a double bond to make eicosatetraenoic acid (C20, 5 double bonds). Additional desaturation, elongation, and oxidation steps finally result in docosahexaenoic acid. Figure 6-3 shows the systematic organic chemistry names and numbering for the first and last structures in this pathway. The systematic name of α-linoleic acid is 9,12,15-octadecadienoic acid, whereas the systematic name of the final product is 4,7,10,13,16,19-docosahexaenoic acid. Counting from the C1 position, as is required in systematic naming, the double bonds that were numbered 9, 12, and 15 in the precursor are now numbered 13, 16, and 19 in the product (9 → 13; 12 → 16; 15 → 19). Repeated many times for PUFAs, these changes in bond position number when counted from the carboxyl carbon make it difficult to track double bonds and, more importantly, fatty acids that are derived from one another. A solution is to number double bonds from the other end of the molecule, taking advantage of the fact that mammals cannot insert double bonds into the methyl end portion of the PUFA chain. Two conventions that are routinely used, which are effectively identical, are the IUPAC (International Union of Pure and Applied Chemistry) “n minus” convention and the omega convention. Examples of these notations are shown in Figure 6-3 for α-linolenic acid (18:3n−3) and docosahexaenoic acid (22:6n−3). Fatty acids with trans or conjugated double bonds do not ordinarily appear at other than trace levels in mammalian tissues (other than in the skin surface lipids), although they may be consumed as part of the diet. Example structures of trans and conjugated unsaturated fatty acids are shown in Figure 6-4. These fatty acids should not be designated with the n− or ω notation, unless further specification is provided. Unlike the situation with common fatty acids, there is no universal specialized notation for these more unusual fatty acids. Either systematic notation or some convenient adaptation of systematic numbering may be used. An example of acceptable notation would be “trans-18:1n−9” to specify the monounsaturated C18 fatty acid with a trans double bond between C9 and C10. For fatty acids with more than one double bond, a “Δ” notation system is frequently used. The superscript Δ precedes the designation of the sites of unsaturation counting from the carboxyl carbon. For instance, 18:3Δ6,9,12 designates γ-linolenic acid. γ-Linolenic acid is also similarly designated as 9,12,15-18:3. Notations in which the individual double bonds are denoted, by counting from the carboxyl end, lend themselves to designation of isomers of γ-linolenic acid, often by adding leading “c” or “t” to specify double bond cis or trans geometry (e.g., c9,t11-octadecadienoic acid, or 18:2Δ c9,t11, for rumenic acid. Stereochemistry is an important property of glycerolipids. The glycerol molecule possesses a plane of symmetry such that the central carbon atom can be considered prochiral, a chemical term referring to a carbon atom with four substituents, three of which are different. A prochiral carbon is not chiral, but substitution of one of the equivalent substituent groups with a fourth, nonequivalent group renders the central carbon chiral. This is the case for the central carbon of glycerol: when non–chemically equivalent moieties are added to the −CH2OH groups, the central carbon becomes chiral. Chirality is very important to biochemical properties and thus must be described unambiguously. Here, the systematic notation of organic chemistry, with rules for designating chiral centers as R or S, leads to even more confusing designations than in the case of fatty acid double bond position. However, a single notation that closely parallels metabolism was introduced in the 1960s and is widely used. As shown in Figure 6-5, glycerol can be positioned so that the top and bottom −CH2OH are oriented with their −OH groups extending to the right and the middle −OH extending to the left. By convention, the positions are referred to as sn-1, sn-2, and sn-3 (top to bottom), with sn being an abbreviation for “stereospecific numbering.” In some contexts, the sn-1 and sn-3 positions are metabolically equivalent and are designated the α positions, with the center sn-2 position then being designated the β position. When non–chemically equivalent groups are added to the sn-1 and sn-3 positions, glycerol becomes chiral. Examples of structures and nomenclature for monoacylglycerols, diacylglycerols, and triacylglycerols are shown in Figure 6-6. Use of the IUPAC-IUB sn nomenclature is generally preferred over the use of the trivial names in most cases, although trivial names are commonly used for simple acylglycerols (e.g., the use of triolein for 1,2,3-tri-cis-9-octadecenoyl-sn-glycerol). A benefit of the sn nomenclature is that it clearly reveals the relationship between the precursor TAG and its DAG and MAG hydrolysis products. For example, the hydrolysis of 1-palmitoyl-2-stearoyl-3-myristoyl-sn-glycerol at the 1 position yields 2-stearoyl-3-myristoyl-sn-glycerol (Figure 6-7). The general structure of the diacylphospholipids is presented in Figure 6-8, and the several classes of common phospholipids and their structures are shown in Figure 6-9. In all cases, a phosphate group is esterified to the sn-3 position of glycerol, and fatty acids are esterified to the sn-1 and sn-2 positions. Most commonly, the sn-2 position is occupied by an unsaturated acyl chain, whereas the sn-1 position is occupied by a saturated chain. However, there are notable exceptions to this generality. For instance, the major surfactant lipid of the lung has 16:0 in both positions, and some of the phospholipids of the retinal photoreceptors have very high concentrations of unsaturated fatty acyl chains in both positions.
Structure, Nomenclature, and Properties of Lipids
The Chemical Classes of Lipids—Their Structure and Nomenclature
Fatty Acids
Saturated Fatty Acids
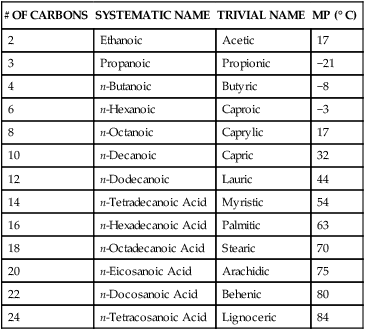
# OF CARBONS
SYSTEMATIC NAME
TRIVIAL NAME
MP (° C)
2
Ethanoic
Acetic
17
3
Propanoic
Propionic
−21
4
n-Butanoic
Butyric
−8
6
n-Hexanoic
Caproic
−3
8
n-Octanoic
Caprylic
17
10
n-Decanoic
Capric
32
12
n-Dodecanoic
Lauric
44
14
n-Tetradecanoic Acid
Myristic
54
16
n-Hexadecanoic Acid
Palmitic
63
18
n-Octadecanoic Acid
Stearic
70
20
n-Eicosanoic Acid
Arachidic
75
22
n-Docosanoic Acid
Behenic
80
24
n-Tetracosanoic Acid
Lignoceric
84
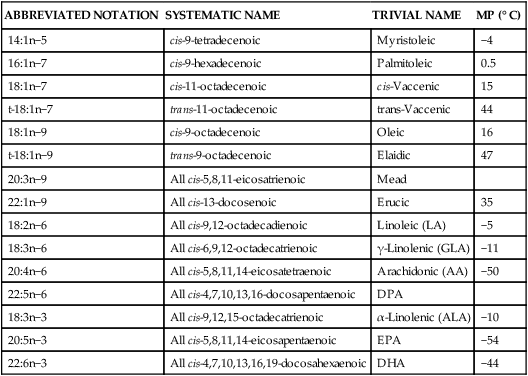
Unsaturated Fatty Acids
ABBREVIATED NOTATION
SYSTEMATIC NAME
TRIVIAL NAME
MP (° C)
14:1n−5
cis-9-tetradecenoic
Myristoleic
−4
16:1n−7
cis-9-hexadecenoic
Palmitoleic
0.5
18:1n−7
cis-11-octadecenoic
cis-Vaccenic
15
t-18:1n−7
trans-11-octadecenoic
trans-Vaccenic
44
18:1n−9
cis-9-octadecenoic
Oleic
16
t-18:1n−9
trans-9-octadecenoic
Elaidic
47
20:3n−9
All cis-5,8,11-eicosatrienoic
Mead
22:1n−9
All cis-13-docosenoic
Erucic
35
18:2n−6
All cis-9,12-octadecadienoic
Linoleic (LA)
−5
18:3n−6
All cis-6,9,12-octadecatrienoic
γ-Linolenic (GLA)
−11
20:4n−6
All cis-5,8,11,14-eicosatetraenoic
Arachidonic (AA)
−50
22:5n−6
All cis-4,7,10,13,16-docosapentaenoic
DPA
18:3n−3
All cis-9,12,15-octadecatrienoic
α-Linolenic (ALA)
−10
20:5n−3
All cis-5,8,11,14-eicosapentaenoic
EPA
−54
22:6n−3
All cis-4,7,10,13,16,19-docosahexaenoic
DHA
−44
Acylglycerols
Prochiral Glycerol
Monoacylglycerols, Diacylglycerols, and Triacylglycerols
Glycerophospholipids
Diacylphospholipids, the Common Phospholipids
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Structure, Nomenclature, and Properties of Lipids