Chapter 46
Structure, Function, and Disorders of the Integument
Sue Ann McCann and Sue E. Huether
Structure and Function of the Skin
Layers of the Skin
The skin is formed of two major layers: (1) a superficial or outer layer of epidermis, and (2) a deeper layer of dermis (the true skin) (Figure 46-1). The subcutaneous layer (hypodermis) is the lowest lying layer of connective tissue that contains macrophages, fibroblasts, fat cells, nerves, fine muscles, blood vessels, lymphatics, and hair follicle roots. Each skin layer contains cells that represent progressive stages of skin cell differentiation and function as the skin grows. These are summarized in Table 46-1.
TABLE 46-1
| LAYER | CELL TYPES | CHARACTERISTICS |
| Epidermis | Most important layer of skin; normally very thin (0.12 mm) but can thicken and form corns or calluses with constant pressure or friction; includes rete pegs that extend into papillary layer of dermis | |
| Stratum corneum | Keratinocytes | Tough superficial sheets of cornified cells |
| Stratum lucidum | Keratinocytes | Clear layers of cells containing eleidin, which becomes keratin as cells move up to the corneum layer; found only in palmoplantar skin |
| Stratum granulosum | Keratinocytes | Lose their nuclei Keratohyalin gives a granular appearance to this layer |
| Stratum spinosum | Keratinocytes Langerhans cells | Polygonal-shaped with spinous processes projecting between adjacent keratinocytes Cells with dendritic processes and immune function |
| Stratum basale (germinativum) | Keratinocytes | Basal layer where keratinocytes divide and move upward to replace cells shed from the surface |
| Melanocytes | Originate in neural crest and migrate to stratum basale and produce melanin | |
| Merkel cells | The function of Merkel cells is not clearly known—have role in light touch | |
| Dermis Papillary layer (thin) Reticular layer (thick) | Macrophages Mast cells Histiocytes Fibroblasts | Irregular connective tissue layer with rich blood, lymphatic, and nerve supply; contains sensory receptors and sweat glands (apocrine, eccrine, sebaceous); macrophages (phagocytic and important for wound healing) and mast cells (release histamine) have immune functions (see Chapter 7) Histiocytes are wandering macrophages that collect pigments and inflammatory debris Generate connective tissue; imported for wound healing |
| Subcutaneous (Hypodermis) | Subcutaneous tissue or superficial fascia of varying thickness that connects the overlying dermis to underlying muscle; contains macrophages, fibroblasts, fat cells, nerves, blood vessels, lymphatics, and hair follicle roots |
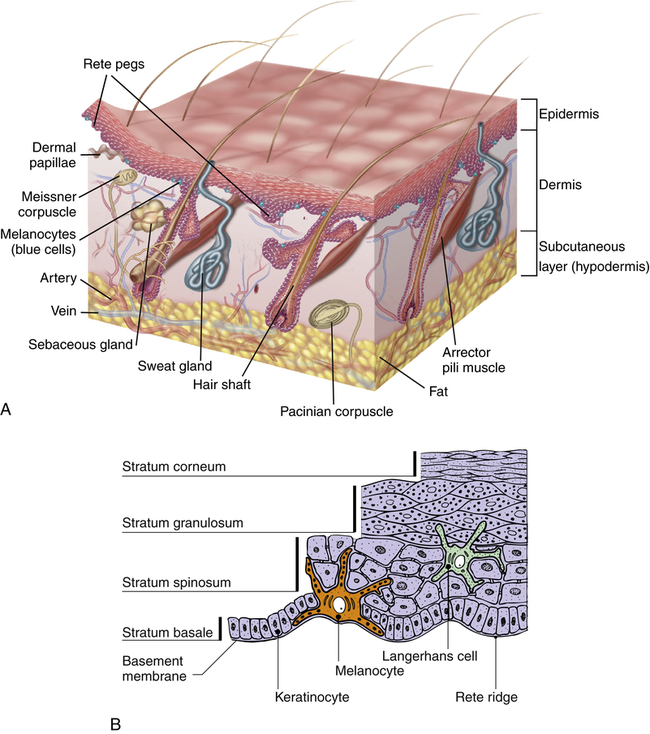
A, Cross section showing major skin structures. B, Layers of the epidermis. (A from Kumar V et al: Robbins & Cotran pathologic basis of disease, ed 8, St Louis, 2009, Saunders; B from Gawkrodger D, Ardern-Jones MR: Dermatology, ed 5, Philadelphia, 2012, Churchill Livingstone.)
Epidermis
The epidermis has three additional types of cells that facilitate its functional characteristics: melanocytes, Langerhans cells, and Merkel cells. The melanocytes are usually located near the base of the epidermis. They synthesize and secrete the pigment melanin with exposure to UV light in response to melanocyte-stimulating hormone (MSH). Melanin in the epidermis provides a shield against UV radiation and determines skin color. Vitiligo is an autoimmune-related loss of melanocytes resulting in the depigmentation of patches of skin (see “Patch” in Table 44-3, p. 1547). Langerhans cells migrate to the epidermis from the bone marrow. Langerhans cells (a type of dendritic cell) and dermal dendritic cells initiate an immune response by presenting processed antigen to T cells, thus providing a defense against environmental antigens.1 Merkel cells are associated with touch receptors and function as slowly adapting mechanoreceptors when stimulated by deformation of the epidermis.
Subcutaneous Layer
Dermal Appendages
The dermal appendages include the nails, hair, sebaceous glands, and the eccrine and apocrine sweat glands. The nails are protective keratinized plates composed of four structural units: (1) the proximal nail fold, (2) the matrix from which the nail grows, (3) the hyponychium (nail bed), and (4) the nail plate (Figure 46-2). Nail growth is continuous throughout life at a rate of 1 mm or less per day.
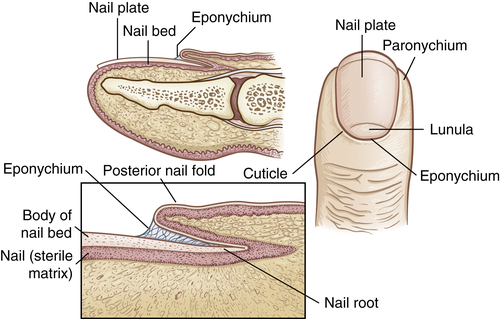
Hair follicles and sebaceous glands are integrated units (see Figure 46-1). Hair color, density, grain, and pattern of distribution have considerable variability and depend on age, gender, and race. Hair follicles arise from the matrix (or bulb) located deep in the dermis. They extend from the dermis at an angle and have an erector pili muscle attached near the mid-dermis that straightens the follicle when contracted, causing the hair to stand up. Hair growth begins in the bulb, with cellular differentiation of stem cells occurring as the hair progresses up the follicle.2 Hair color is determined by melanin-secreting follicular melanocytes. Hair is fully hardened, or cornified, by the time it emerges at the skin surface. Hair growth is cyclic, with periods of growth and rest that vary over different body surfaces.
Aging and Skin Integrity
Many age-associated changes in the skin are generalized and readily observable. Genetics and cumulative environmental factors, including UV radiation from sun exposure (photoaging), inflammatory responses, tobacco smoke, and gravity contribute to cutaneous changes with aging.3,4 Structurally the skin becomes thinner, less elastic, drier, and wrinkled with a change in pigmentation. The cellular alterations contributing to the changes include a flattening of the dermoepidermal junction with a shortening and decrease in the number of capillary loops. There are fewer melanocytes, resulting in decreased protection against UV radiation. A significant decrease in the number of Langerhans cells reduces the skin’s immune response with aging. The thickness of the dermis also decreases and accounts for the translucent, paper-thin quality of the skin. Loss of the rete pegs gives the skin a smooth, shiny appearance.5
The decreased vasculature and lymphatic drainage contribute to loss of barrier protection and the atrophy of eccrine, apocrine, and sebaceous glands, all of which promote dry skin. Loss of elastin fibers is associated with wrinkling. Collagen fibers become fragmented, and fibroblasts decrease in number, resulting in a decreased ability of the skin to stretch and regain shape. Decreased cell proliferation, diminished blood supply, and depressed immune responses also delay wound healing in aging skin. Changes in hair color and distribution also occur. Graying is caused by loss of melanocytes from hair bulbs, and thinning occurs from a gradual decline in the number of hair follicles and growth of finer hair.6
Epidermal cells change shape, and the barrier function of the stratum corneum is reduced. There is increased permeability and decreased clearance of toxic oxidative substances from the dermis. The accumulation of such substances is related to decreased vascularity and can cause skin irritation. Temperature regulation is compromised in older adults, with an increased risk for heat stroke and hypothermia. Loss of cutaneous vasomotion and subcutaneous fat, reduced vascularity, and decreased eccrine sweat production are contributing factors.7 The pressure and touch receptors and free nerve endings decrease in number and reduce sensory perception. With aging many of the protective functions of the skin decrease, whereas the likelihood of infection and delay in wound healing increase.8
Tests of Skin Function
Diagnostic evaluations of skin disorders often can be completed by gathering historical information, performing a physical examination, and observing the distribution and characteristics of the presenting lesions. Additional diagnostic studies are summarized in Table 46-2.
TABLE 46-2
SUMMARY OF SKIN DIAGNOSTIC PROCEDURES
| TEST | PURPOSE |
| Dermoscopy | Magnified illumination of the skin using a liquid medium and transparent plate to examine skin lesions |
| Skin biopsy | Histologic examination of tissue to determine differential diagnosis of cellular structure (i.e., benign growths vs. carcinoma, chronic infections, blistering diseases, and vasculitis) |
| Microscopic immunofluorescence | Identification of antibodies, immunoglobulins, and complement components for diseases such as pemphigus, vasculitis, and discoid lupus erythematosus using fluorescent light on slide-mounted biopsy specimens |
| Gram stain | Differentiation of gram-positive from gram-negative bacteria according to stain absorption |
| Culture | Identification of chronic bacterial and fungal infections by incubating skin specimens in culture media |
| Wood lamp examination | Examination of skin or hair to identify fungus that fluoresces bright yellow-green under ultraviolet light |
| Patch and scratch tests | Application of suspected allergens to skin by patch or scratch for evaluation of immune system responses to known allergens and evaluation of cell-mediated immune function (Candida albicans, skin fungus, chemicals, aeroallergens, and foods) |
| Skin scrapings | Application of potassium hydroxide and low heat to skin scrapings on a glass slide to identify dermatophytes and C. albicans |
| Side lighting | Indirect lighting of the skin using light to the side of the lesions to evaluate patterns of depression and elevation of skin lesions |
| Diascopy | Use of glass or clear plastic pressed on the skin to differentiate erythema caused by dilated capillaries (blanching) from extravasation of blood (no blanching) |
| Tzanck smear | A microscopic examination of cellular material from skin lesions to help diagnose vesicular diseases, including herpes simplex virus and varicella-zoster virus |
Clinical Manifestations of Skin Dysfunction
Lesions
Lesions of the skin are readily observable and easily assessed for distribution and structure. Identification of the morphologic structure, including a differentiation between the primary and secondary lesions, and the general appearance of the skin is critical. The physical exam, in combination with a thorough health history, is essential to identify the underlying pathophysiology. Table 46-3 describes and illustrates the primary lesions of the skin and Table 46-4 describes and illustrates the secondary lesions of the skin. Special skin lesions are described in Table 46-5.
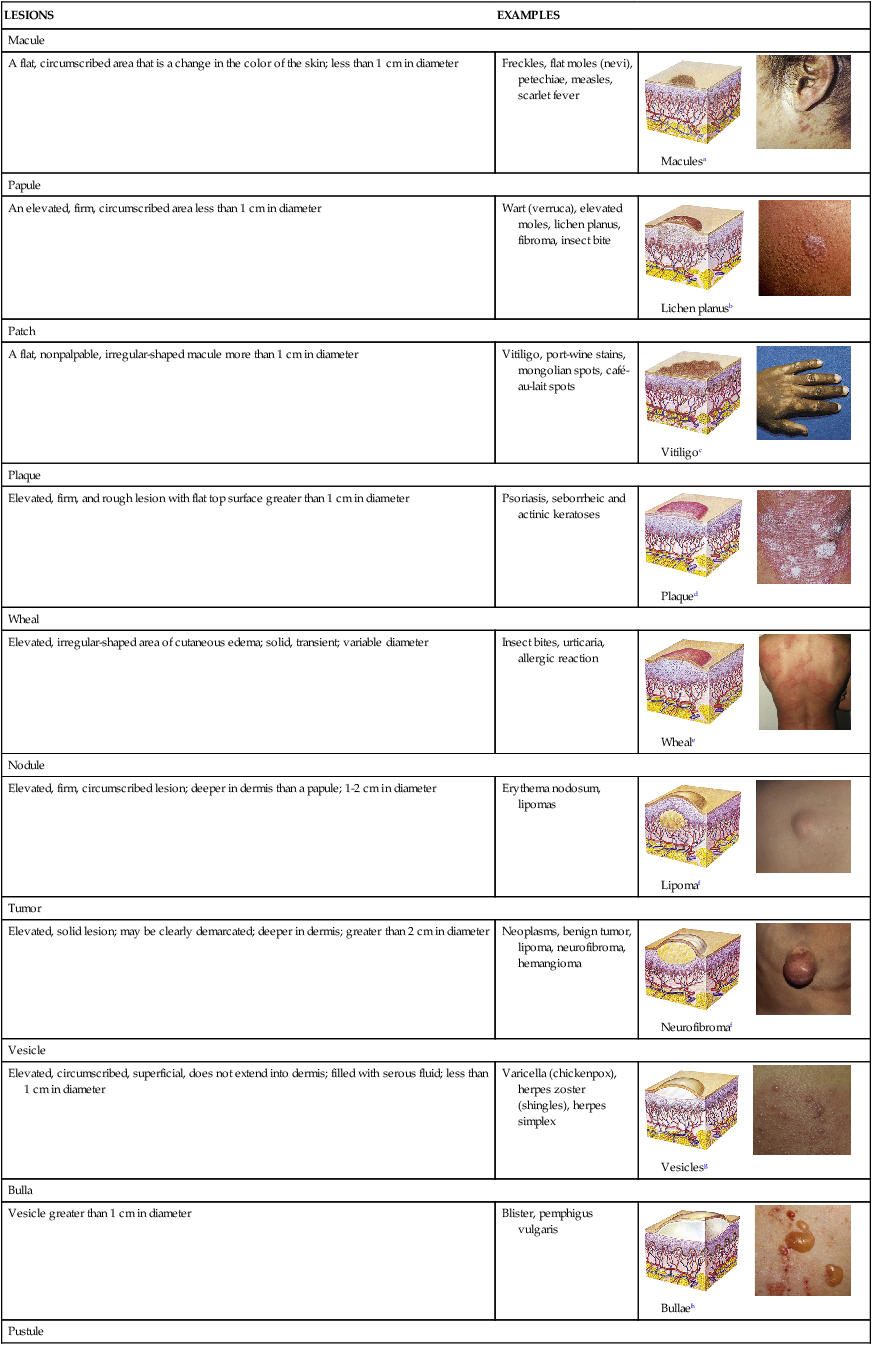
TABLE 46-3
| LESIONS | EXAMPLES | |
| Macule | ||
| A flat, circumscribed area that is a change in the color of the skin; less than 1 cm in diameter | Freckles, flat moles (nevi), petechiae, measles, scarlet fever | 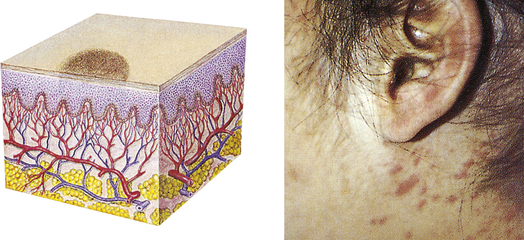 Maculesa |
| Papule | ||
| An elevated, firm, circumscribed area less than 1 cm in diameter | Wart (verruca), elevated moles, lichen planus, fibroma, insect bite | 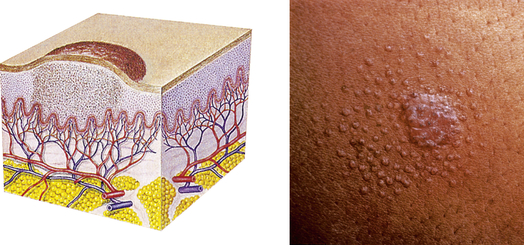 Lichen planusb |
| Patch | ||
| A flat, nonpalpable, irregular-shaped macule more than 1 cm in diameter | Vitiligo, port-wine stains, mongolian spots, café-au-lait spots | 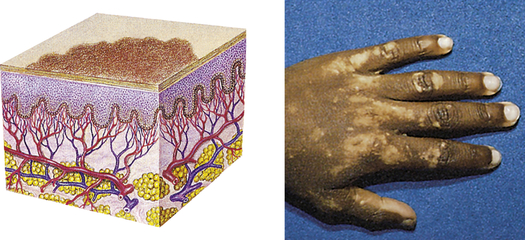 Vitiligoc |
| Plaque | ||
| Elevated, firm, and rough lesion with flat top surface greater than 1 cm in diameter | Psoriasis, seborrheic and actinic keratoses | 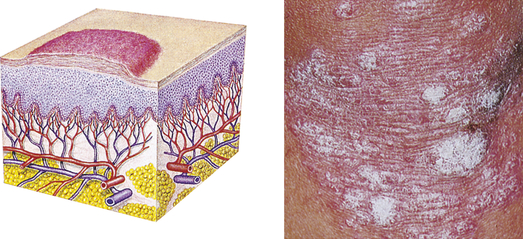 Plaqued |
| Wheal | ||
| Elevated, irregular-shaped area of cutaneous edema; solid, transient; variable diameter | Insect bites, urticaria, allergic reaction | 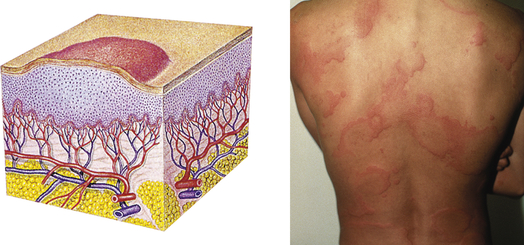 Wheale |
| Nodule | ||
| Elevated, firm, circumscribed lesion; deeper in dermis than a papule; 1-2 cm in diameter | Erythema nodosum, lipomas | 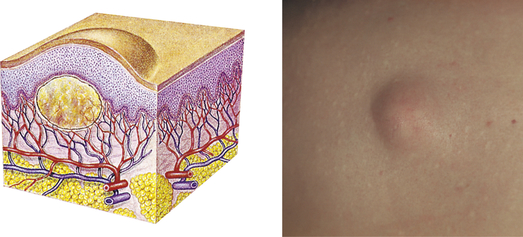 Lipomaf |
| Tumor | ||
| Elevated, solid lesion; may be clearly demarcated; deeper in dermis; greater than 2 cm in diameter | Neoplasms, benign tumor, lipoma, neurofibroma, hemangioma | 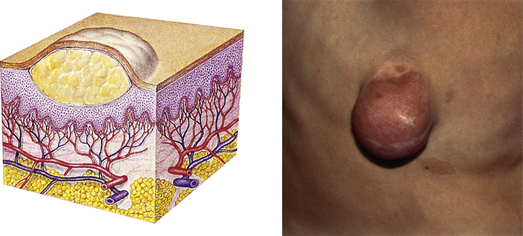 Neurofibromaf |
| Vesicle | ||
| Elevated, circumscribed, superficial, does not extend into dermis; filled with serous fluid; less than 1 cm in diameter | Varicella (chickenpox), herpes zoster (shingles), herpes simplex | 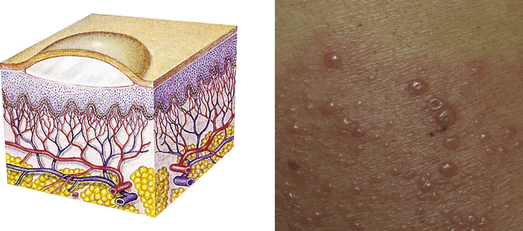 Vesiclesg |
| Bulla | ||
| Vesicle greater than 1 cm in diameter | Blister, pemphigus vulgaris | 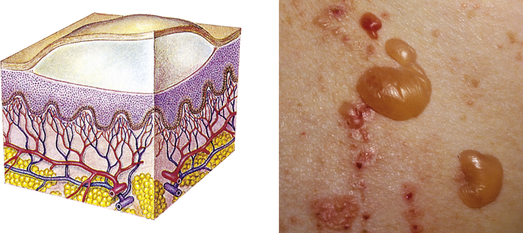 Bullaeh |
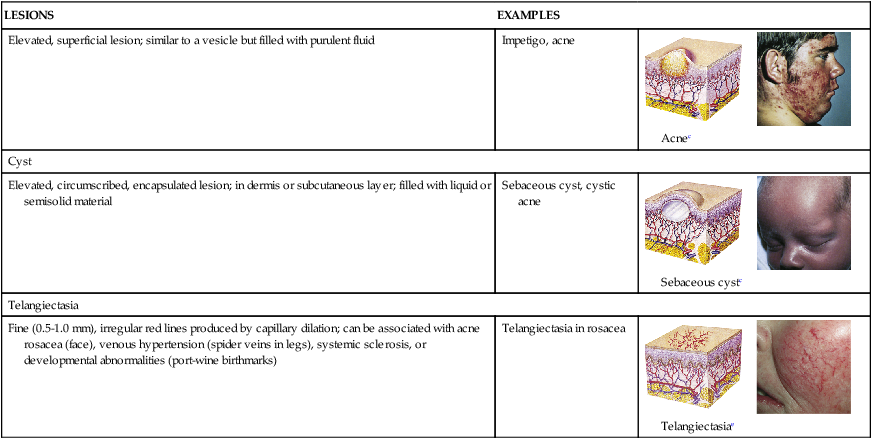
| Pustule | ||
| Elevated, superficial lesion; similar to a vesicle but filled with purulent fluid | Impetigo, acne | 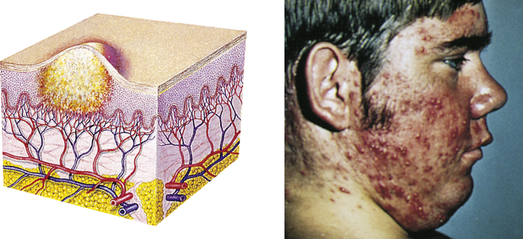 Acnec |
| Cyst | ||
| Elevated, circumscribed, encapsulated lesion; in dermis or subcutaneous layer; filled with liquid or semisolid material | Sebaceous cyst, cystic acne | 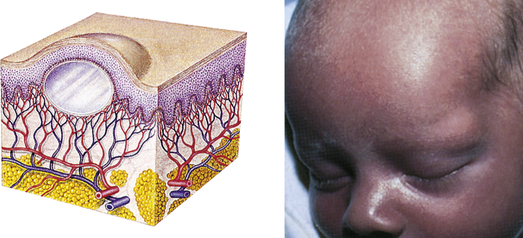 Sebaceous cystc |
| Telangiectasia | ||
| Fine (0.5-1.0 mm), irregular red lines produced by capillary dilation; can be associated with acne rosacea (face), venous hypertension (spider veins in legs), systemic sclerosis, or developmental abnormalities (port-wine birthmarks) | Telangiectasia in rosacea | 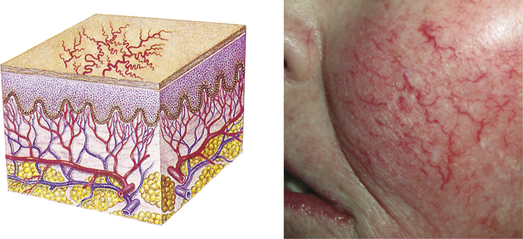 Telangiectasiae |
aFarrar WE et al: Infectious diseases, ed 2, London, 1992, Gower.
bJames WD, Berger T, Elston DMD: Andrews’ diseases of the skin, ed 11, Philadelphia, 2011, Saunders.
cWeston WL, Lane AT: Color textbook of pediatric dermatology, ed 3, Philadelphia, 2002, Mosby.
dHabif TP: Clinical dermatology: a color guide to diagnosis and therapy, ed 5, Philadelphia, 2010, Mosby.
eBolognia JL, Jorizzo JL, Schaffer JV: Dermatology, ed 3, Philadelphia, 2012, Saunders.
fWeston WL, Lane AT, Morelli JG: Color textbook of pediatric dermatology, ed 4, Philadelphia, 2007, Mosby.
gBlack MM et al: Obstetric and gynecologic dermatology, ed 3, Philadelphia, 2008, Mosby.
hMarks JG, Miller JJ: Lookingbill & Marks’ principles of dermatology, ed 4, London, 2006, Saunders.
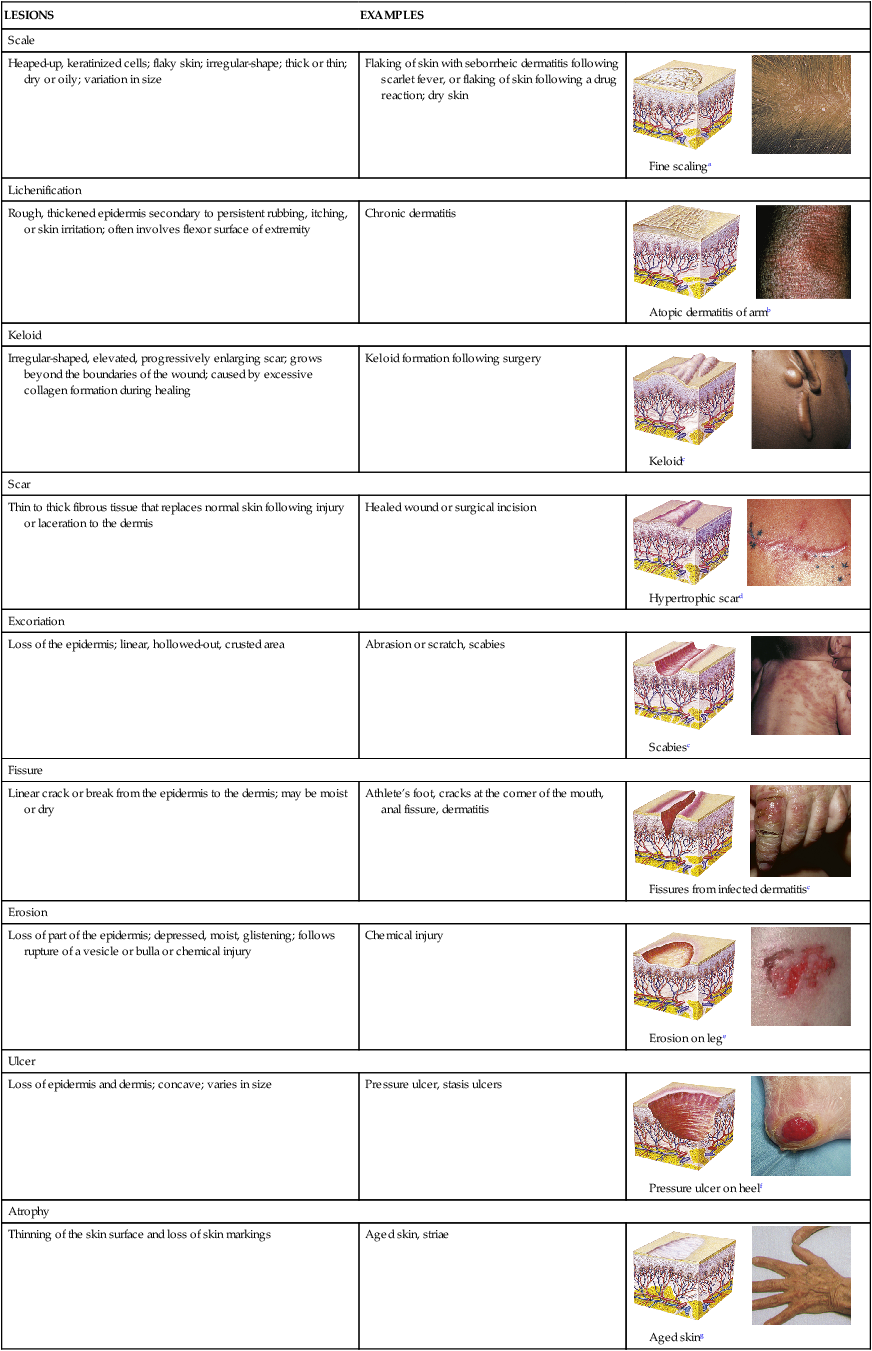
TABLE 46-4
| LESIONS | EXAMPLES | |
| Scale | ||
| Heaped-up, keratinized cells; flaky skin; irregular-shape; thick or thin; dry or oily; variation in size | Flaking of skin with seborrheic dermatitis following scarlet fever, or flaking of skin following a drug reaction; dry skin | 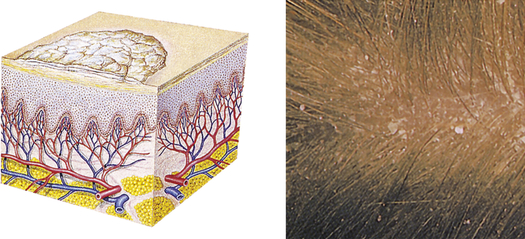 Fine scalinga |
| Lichenification | ||
| Rough, thickened epidermis secondary to persistent rubbing, itching, or skin irritation; often involves flexor surface of extremity | Chronic dermatitis | 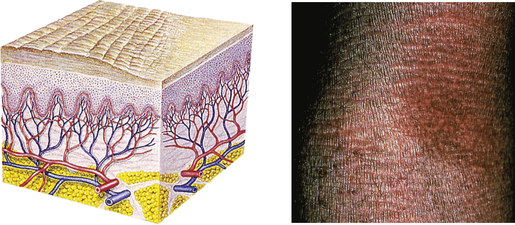 Atopic dermatitis of armb |
| Keloid | ||
| Irregular-shaped, elevated, progressively enlarging scar; grows beyond the boundaries of the wound; caused by excessive collagen formation during healing | Keloid formation following surgery | 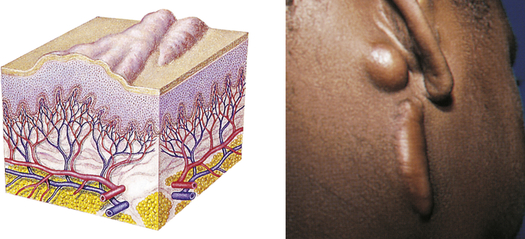 Keloidc |
| Scar | ||
| Thin to thick fibrous tissue that replaces normal skin following injury or laceration to the dermis | Healed wound or surgical incision | 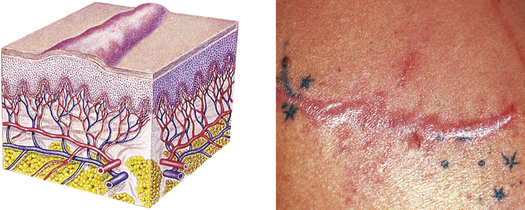 Hypertrophic scard |
| Excoriation | ||
| Loss of the epidermis; linear, hollowed-out, crusted area | Abrasion or scratch, scabies | 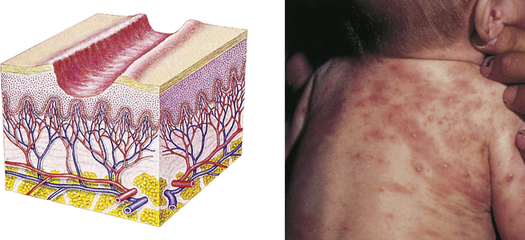 Scabiesc |
| Fissure | ||
| Linear crack or break from the epidermis to the dermis; may be moist or dry | Athlete’s foot, cracks at the corner of the mouth, anal fissure, dermatitis | 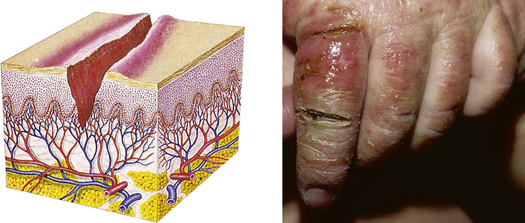 Fissures from infected dermatitisc |
| Erosion | ||
| Loss of part of the epidermis; depressed, moist, glistening; follows rupture of a vesicle or bulla or chemical injury | Chemical injury | 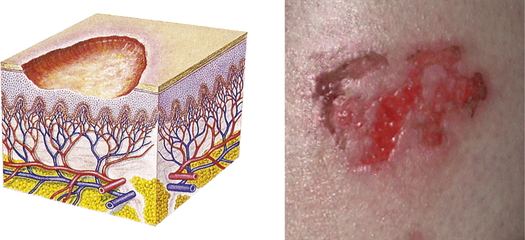 Erosion on lege |
| Ulcer | ||
| Loss of epidermis and dermis; concave; varies in size | Pressure ulcer, stasis ulcers | 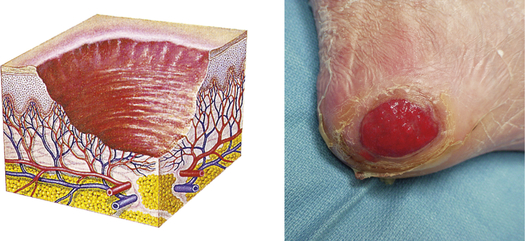 Pressure ulcer on heelf |
| Atrophy | ||
| Thinning of the skin surface and loss of skin markings | Aged skin, striae | 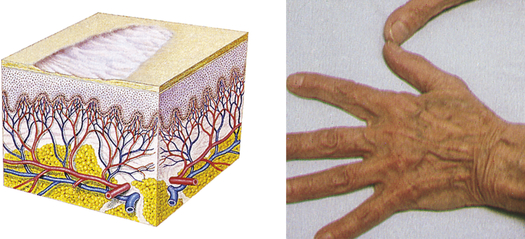 Aged sking |
aBaran R, Dawber RR, Levene GM: Color atlas of the hair, scalp, and nails, St Louis, 1991, Mosby.
bJames WD, Berger T, Elston DMD: Andrews’ diseases of the skin, ed 11, Philadelphia, 2011, Saunders.
cWeston WL, Lane AT, Morelli JG: Color textbook of pediatric dermatology, ed 4, Philadelphia, 2007, Mosby.
dNouri K, Leal-Khouri S: Techniques in dermatologic surgery, Philadelphia, 2003, Mosby.
eBolognia JL, Jorizzo JL, Schaffer JV: Dermatology, ed 3, Philadelphia, 2012, Saunders.
fRobinson JK et al: Surgery of the skin, ed 2, Philadelphia, 2010, Mosby.
gSeidel HM et al: Mosby’s guide to physical examination, ed 7, St Louis, 2011, Mosby.
TABLE 46-5
| TYPE | CLINICAL MANIFESTATIONS |
| Comedone | A plug of sebaceous and keratin material lodged in the opening of a hair follicle; an open comedone has a dilated orifice (blackhead), and a closed comedone has a narrow opening (whitehead) |
| Burrow | A narrow, raised, irregular channel caused by a parasite |
| Petechiae | A circumscribed area of blood less than 0.5 cm in diameter |
| Purpura | A circumscribed area of blood greater than 0.5 cm in diameter |
Pressure Ulcers
Pressure ulcers are ischemic ulcers resulting from unrelieved pressure, shearing forces, friction, and moisture. Pressure that consistently interrupts arterial and venous blood flow to and from the skin or deeper tissue is the most significant cause.9 The term decubitus ulcer refers to an ulcer or pressure sore that results when an individual lies or sits in one position for a long time. The more general terms of pressure sore or ulcer are used here. Risk factors for pressure ulcers are summarized in Box 46-1.
Most individuals with darkly pigmented skin are at greater risk for developing pressure ulcers because early signs of skin damage may not be clearly visible.10 Pressure sores usually develop over bony prominences: the sacrum, heels, ischia, and greater trochanters are the most common sites. Continuous pressure on tissue between the bony prominence and a resistant outside surface distort capillaries and occlude the blood flow and oxygen supply. Several scales are available for predicting pressure sore risk; the Braden Scale is one of those most frequently used.11–13 One classification of pressure ulcer grades or stages is summarized as follows14:
I Nonblanchable erythema of intact skin usually over bony prominence; darkly pigmented skin may not have visible blanching
II Partial-thickness skin loss (erosion or blistering) involving epidermis or dermis presenting as a shallow open ulcer with a red-pink wound bed, without slough
III Full-thickness skin loss involving damage or necrosis of subcutaneous tissue that may extend to, but not through, underlying fascia
IV Full-thickness tissue loss with exposure of muscle, bone, or supporting structures (tendons or joint capsules); can include undermining and tunneling
Superficial damage results in a layer of dead tissue that appears as a blister, erosion, or nonblanchable red/darkened skin or as a reddish blue discoloration when there is deeper tissue damage. Superficial sores are more common on the sacrum as a result of shearing or friction forces (forces parallel to the skin). Deep sores develop closer to the bone as a result of tissue distortion and vascular occlusion from pressure that is perpendicular to the tissue (over the heels, trochanter, and ischia) (Figure 46-3).
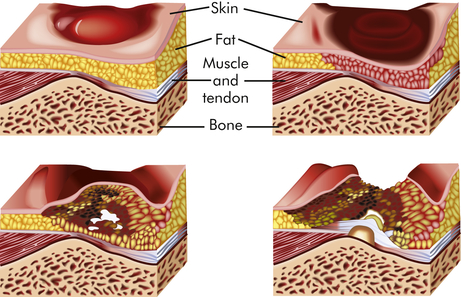
Sustained pressure over a bony prominence compresses the tissue and reduces blood flow, resulting in progressive ischemia and necrosis of tissue.
The primary goal for those at risk for pressure ulcers is prevention, including frequent skin assessment with repositioning and turning; use of pressure reduction surfaces and specialty beds; elimination of incontinence, moisture, and drainage; and maintenance of fluid, protein, and caloric intakes.15,16 Superficial ulcers should be covered with flat, moisture-retaining dressings that cannot wrinkle and cause increased pressure or friction; multiple options are available.17 Successful healing requires continued adequate relief of pressure; débridement of dead tissue; use of specialty wound care products (dressings, foams, gels, cleansers); and repair with skin flaps for large, deep ulcers. Negative pressure wound healing is a useful technology in the treatment of stage III/IV pressure ulcers.18,19 Infection requires treatment with antibiotics and pain should be controlled.20 Randomized controlled trials are needed to determine the best methods for treating pressure ulcers.21,22
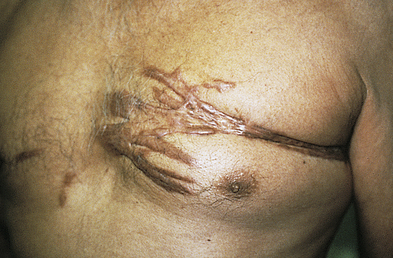
Keloids and Hypertrophic Scars
Keloids are rounded, firm, elevated scars with irregular clawlike margins that extend beyond the original site of injury. Hypertrophic scars are elevated erythematous fibrous lesions that do not expand beyond the border of injury. Both lesions are genetically influenced and caused by abnormal wound healing with excessive fibroblast activity and collagen formation during dermal connective tissue repair.23,24 A familial tendency for keloid formation has been found and both autosomal recessive and autosomal dominant inheritance patterns have been reported.25 Genes regulating fibroblasts may be up- or down-regulated in keloid tissue.26
Keloids are most common in darkly pigmented skin types and burn scars (see Chapter 48). Excessive or poorly aligned tension on a wound, introduction of foreign material into the skin, and the occurrence of certain types of trauma (e.g., burns) are provocative factors. At risk are the shoulders, back, chin, ears, and lower legs. Keloids can appear within weeks to months after a stable scar has formed. Individuals 10 to 30 years of age develop lesions much more commonly than do children before puberty or older adults. Hypertrophic scars usually regress within a year.
The amount of type III collagen is increased with keloids. The increased synthesis of collagen is associated with interleukin-6 (IL-6) signaling and dermal fibroblasts that have high metabolic and mitotic rates. There is aberrant expression of transforming growth factor-beta (TGF-β) and platelet-derived growth factor (PDGF).27
Myofibroblasts, cells with characteristics of fibroblasts and smooth muscle cells, are the principal cells in keloids. Collagenase activity in keloids is normal or increased, but the collagen may be protected from degradation by proteoglycan, a glycoprotein present in connective tissue that serves as a binding (cementing) material, and by specific inhibitors of proteolytic enzymes.28 Keloids first appear as pink or red, firm, well-defined rubbery plaques that persist for several months after trauma. Later, uncontrolled overgrowth causes extension beyond the site of the original wound and the tumor becomes smoother, irregularly shaped, hyperpigmented, and harder with clawlike prolongations (Figure 46-4).
Keloids are the most extreme example of cutaneous scarring and the most difficult to treat. Preventive measures such as avoidance of unnecessary elective surgeries, recognition of familial tendency, and early detection are of paramount importance. Standard management of keloids and hypertrophic scars includes intralesional corticosteroids, cryotherapy, radiotherapy, and surgical/laser procedures whereas intralesional injections of interferon, bleomycin, and 5-fluorouracil (5-FU) are emerging treatments.29 There remains a need for research to improve treatment outcome.30,31
Pruritus
Multiple stimuli can produce itching and there is interaction between itch and pain sensations. Peripheral pruritogenic (itch) mediators include histamine, neuropeptides, serotonin, prostaglandins, bradykinin, substance P, opioids, acetylcholine, interleukin-31, and nerve growth factor. Itch sensation is thought to be carried by specific unmyelinated C nerve fibers and thinly myelinated Aδ nerve fibers. Specific spinal pathways carry itch sensations through the spinal cord to the brain.32 These nerve fibers also may interact with dermal eosinophils, lymphocytes, and mast cells.33
Central nervous system mechanisms also can modulate itching, which is less perceptible when the mind is distracted by other stimuli. How the central nervous system influences the itch sensation is unclear. Neuropathic itch (itch without pruritogenic stimuli) is related to pathology along an afferent pathway (i.e., postherpetic neuropathy).34 Psychogenic itch is associated with psychologic disorders (e.g., depression and obsessive-compulsive disorder).35
Chronic itching is an unpleasant sensation that may or may not be relieved by scratching—often done so intensely that trauma to the skin occurs, resulting in infection, skin thickening (lichenification), and scarring. Both central and peripheral sensitization occurs.35 Some individuals become so distraught with the constant irritation that they apply heat and/or ice with enough intensity and duration to produce skin injury. Management of itching is challenging and depends on the cause; the primary condition must be treated. Both topical and systemic therapies are used.36,37
Disorders of the Skin
Inflammatory Disorders
Allergic Contact Dermatitis
Allergic contact dermatitis is a common form of T-cell–mediated or delayed hypersensitivity (type IV).38 (See Chapter 9 for various types of allergic responses.) Genetic susceptibility involves several genes including loss-of-function mutations in the gene encoding the epidermal protein filaggrin.39 Allergens (e.g., microorganisms, chemicals, foreign proteins, drugs, metals, latex) can form the sensitizing antigen; contact with poison ivy is a common example (Figure 46-5). The response is a reaction to irritants with release of cytokines, chemokines, and cytotoxins from keratinocytes, dendritic cells (Langerhans cells), and natural killer cells. When the allergen comes into contact with the skin the allergen is bound to a carrier protein, forming a hapten-specific sensitizing antigen. Langerhans cells process the antigen and carry it to T cells that then become sensitized to the antigen, releasing cytokines and chemokines and leading to leukocyte infiltration and inflammation.40 Latex allergy can be either a type IV hypersensitivity reaction to chemicals used in latex rubber processing or a type I immediate hypersensitivity reaction with IgE antibodies formed in response to latex rubber protein.41
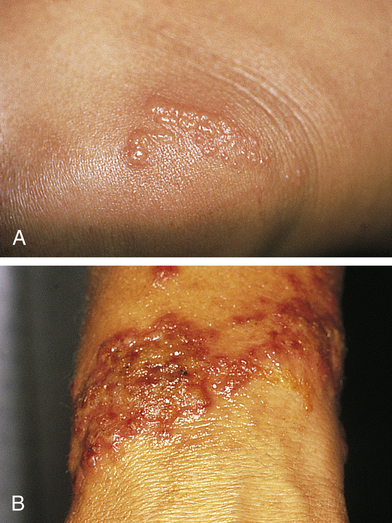
A, Poison ivy on knee. B, Poison ivy dermatitis. (Courtesy Department of Dermatology, School of Medicine, University of Utah.)
In delayed hypersensitivity, several hours pass before an immunologic response is apparent. The T cells play an important role because they differentiate and secrete lymphokines that affect macrophage movement and aggregation, coagulation, and other inflammatory responses (see Chapter 9). Sensitization usually develops with first exposure to the antigen, and symptoms of dermatitis occur with reexposure.
The manifestations of allergic contact dermatitis include erythema and swelling with pruritic (itching) vesicular lesions in the areas of allergen contact. The pattern of distribution provides clues to the source of the antigen (e.g., hands exposed to chemical solutions or boundaries from rings and bracelets). Patch tests with specific antigens may assist with diagnosis. Removal of the allergen is necessary for resolution of the inflammatory response and tissue repair. Topical or systemic steroids, as well as other symptomatic treatment, may be required depending on the severity of the lesion.42
Irritant Contact Dermatitis
Irritant contact dermatitis is a common nonimmunologically-mediated inflammation of the skin. The intensity of the inflammation is related to the concentration of the irritant, exposure time, disruption of the skin barrier, and age.43 Irritation can occur from almost anything, especially if the epidermal barrier is compromised in any way (Box 46-2). The skin lesions are similar in appearance to allergic contact dermatitis. Removing the source of irritation and using topical agents (i.e., corticosteroids and petroleum-based emollients) and nonirritating soaps constitute effective treatment.
Stasis Dermatitis
Stasis dermatitis usually occurs on the legs as a result of venous stasis and edema. The disorder is associated with varicosities (incompetent venous valves), phlebitis, and vascular trauma. Pooling of venous blood traps leukocytes that may release proteolytic enzymes. Increased venous pressure widens interendothelial pores with deposition of red blood cells, fibrin, and other macromolecules, making them unavailable for repair and promoting inflammation.45 Edema evolves to erythema and pruritus with progression to scaling, petechiae, and hyperpigmentation. Progressive lesions become ulcerated (stasis ulcers), particularly around the ankles and tibia (Figure 46-6).
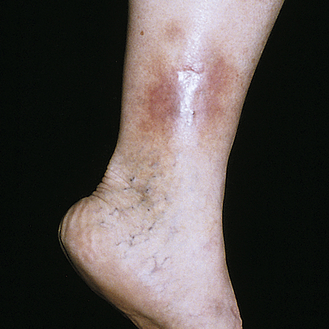
Treatment includes elevating the legs as often as possible, refraining from wearing tight clothes around the legs, and avoiding standing for long periods. Defined infections are treated with antibiotics. Chronic lesions with ulceration are treated with moist dressings, external compression garments, and vein ablation surgery.46
Seborrheic Dermatitis
Seborrheic dermatitis is a common chronic inflammation of the skin involving the scalp, eyebrows, eyelids, ear canals, nasolabial folds, axillae, chest, and back (Figure 46-7). In infants it is known as cradle cap. The cause is unknown, but genetic predisposition, the production of specific phospholipases from the presence of Malassezia yeasts, and immune responses are implicated.47,48 The lesions appear from infancy to old age, with periods of remission and exacerbation. The lesions appear as greasy, scaly, white, or yellowish inflammatory plaques in sebaceous areas with mild pruritus. Mild cases are treated with shampoos containing sulfur, salicylic acid, or tar. Ketoconazole has antifungal and anti-inflammatory effects and has been used with success along with topical calcineurin inhibitors. Corticosteroid applications are useful for suppression of severe symptoms but should not be used for maintenance therapy.49
Papulosquamous Disorders
Psoriasis
Psoriasis is a chronic, relapsing, proliferative, inflammatory disorder that involves the skin, scalp, and nails and can occur at any age. The disease affects about 1% to 8% of the world population and is more common in countries north of the equator.50 The onset is generally established by 20 years of age. A family history of psoriasis is common. The genetic mechanisms are complex and the human leukocyte antigen (HLA)-Cw6 allele (PSORS1) is a major susceptibility gene that affects skin barrier function and innate immune responses.51 Psoriasis is a T helper (Th) cell–mediated autoimmune disease. The interleukin (IL)-23/T helper (Th) 17 cell axis plays an important role in the pathogenesis of psoriasis.52 Inflammatory cytokines involved include tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), IL-6, IL-12, IL-15, IL-17, IL-20, IL-21, and IL-22 derived from activated T cells, B cells, dendritic cells, and macrophages.53
The dermis and epidermis are thickened with keratinocyte hyperproliferation, altered keratinocyte differentiation, expanded dermal vasculature, infiltration of neutrophils and lymphocytes, and inflammation.54 The turnover time for shedding the epidermis is decreased from the normal 26 to 30 days to 3 to 4 days. There are increased numbers of germinative cells and an increase in transit time of cells through the dermis. The rapid cellular proliferation does not allow time for cell maturation and keratinization to occur, resulting in a thickened epidermis and plaque formation. The loosely cohesive keratin gives the lesion a silvery, scaly appearance. There is often capillary dilation and increased vascularization to accommodate the increased cell metabolism. The increased vascularity causes erythema.
The types of psoriasis include plaque (psoriasis vulgaris), inverse, guttate, pustular, and erythrodermic. Plaque psoriasis is the most common and affects 80% to 90% of individuals with psoriasis. The disease can be mild, moderate, or severe, depending on the size, distribution, and inflammation of the lesions. Early onset psoriasis is an inflammatory lesion with epidermal hyperproliferation and the presence of activated T lymphocytes. The typical lesion of plaque psoriasis is a well-demarcated, thick, silvery, scaly, erythematous plaque surrounded by normal skin (Figure 46-8). Initial lesions usually develop insidiously as small erythematous papules that enlarge and coalesce into larger inflammatory lesions that can be painful. The lesions are commonly located on the scalp, elbows, and knees and at sites of trauma. The scales are usually loosely adherent and may cause small bleeding points when removed. Inverse psoriasis is rare and involves lesions that develop in skinfolds (i.e., axilla or groin). They are large, smooth, dry, and deep red. The clinical course of psoriasis is characterized by remissions and exacerbations. Psoriasis may be triggered by drugs including antimalarials, lithium, nonsteroidal anti-inflammatories, and beta-blockers.55
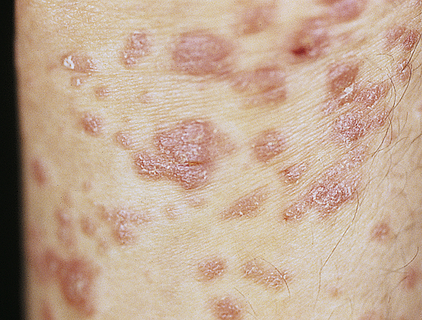
Typical oval plaques with well-defined borders and silvery scale. (Courtesy Department of Dermatology, School of Medicine, University of Utah.)
In guttate psoriasis, small papules (1 to 10 mm) appear suddenly on the trunk and extremities (Figure 46-9). The lesions may appear a few weeks after a streptococcal respiratory tract infection and are more common in children. Guttate psoriasis may resolve spontaneously in weeks or months. Pustular psoriasis appears as blisters of noninfectious pus (collections of neutrophils) that develop over areas of plaque psoriasis. Erythrodermic (exfoliative) psoriasis is characterized by widespread red, scaling lesions that cover a large body surface area (BSA) and is often accompanied by itching or pain associated with constitutional symptoms (fever, chills, fatigue) and skin infections.
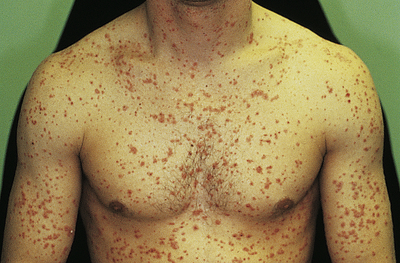
Numerous uniformly small lesions may abruptly occur after streptococcal pharyngitis. (Courtesy Department of Dermatology, School of Medicine, University of Utah.)
Psoriatic arthritis (PsA) and ankylosing spondylitis are associated with the proinflammatory cytokines that cause psoriatic skin lesions. Joints of the hands, feet, knees, and ankles are involved, and 5% to 50% of individuals with psoriasis have seronegative joint involvement. Psoriatic arthritis mutilans involves pronounced bone destruction and there is greater risk of cardiovascular disease with PsA.56,57 Psoriatic nail disease can occur in all psoriasis subtypes with pitting, onycholysis, subungual hyperkeratosis, and nail plate dystrophy. Psoriasis also is a risk factor for a number of comorbidities including inflammatory bowel disease; metabolic syndrome, including hypertension insulin resistance, dyslipidemias, and abdominal obesity; and increased risk for atherosclerosis and cardiovascular disease that is independent of traditional risk factors for these diseases.58,59
Treatment is individualized and related to maintaining skin moisture, reducing epidermal cell turnover and itching, and improving immunomodulation. Mild lesions are usually treated with emollients, keratolytic agents, and corticosteroids. Moderate lesions may respond to UVB light, tar preparations, or a combination of both, and to methotrexate, cyclosporine A, and acitretin. Moderate to severe disease is responsive to the photosensitizing agent oral psoralen in combination with UVA light (PUVA), although skin cancer risk (melanoma and squamous cell) with cumulative dose remains a valid concern.60 Vitamin D3 (calcitriol) is used to reduce epidermal proliferation. Moderate to severe disease is the indication for biologic treatment including TNF-α blockers (adalimumab, etanercept, infliximab) and interleukin (IL)-12/-23 inhibitors (ustekinumab). Clinical trials are evaluating new agents.61,62
Pityriasis Rosea
Pityriasis rosea is a benign self-limiting inflammatory disorder that occurs more often in young adults, with seasonal peaks in the spring and fall. This is a benign disorder except when contracted during pregnancy, when risks are increased for miscarriage and premature delivery.63 The cause is unknown but is thought to be associated with a virus (e.g., human herpesvirus 6 [HHV-6] and HHV-7) because of the timing and clustering of the outbreaks.64 Pityriasis rosea begins as a single lesion known as a herald patch (Figure 46-10) that is circular, demarcated, and salmon-pink; is approximately 3 to 4 cm in diameter; and is usually located on the trunk. Early lesions are macular and papular; secondary lesions develop within 14 to 21 days and extend over the trunk and upper part of the extremities. Lesions are rarely located on the face. They emerge as small erythematous papules that expand into characteristic oval lesions. There may be few or hundreds of lesions. The pattern of distribution follows the skin lines around the trunk and resembles a drooping pine tree. As scales flake off from the margin of the lesions, a collarette pattern is formed. Itching is the most common symptom. Headache, fatigue, or sore throat may precede the development of the lesions.
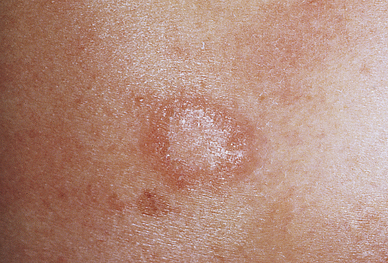
A collarette pattern has formed around the margins. (Courtesy Department of Dermatology, School of Medicine, University of Utah.)
The diagnosis of pityriasis rosea is made by the clinical appearance of the lesion. Secondary syphilis, psoriasis, drug eruption, nummular eczema, and seborrheic dermatitis are among the differential diagnosis considerations. The disorder is usually self-limiting and resolves in a few months with symptomatic treatment for pruritus or cosmetic concerns. UV light (with some risk for hyperpigmentation), antihistamines, or topical corticosteroids may be used to control itching. Erythromycin and acyclovir also may be used for treatment.65
Lichen Planus
Lichen planus (LP) is a benign, autoimmune inflammatory disorder of the skin and mucous membranes with multiple clinical variations. The cause is unknown, but T cells, adhesion molecules, inflammatory cytokines, perforin, and antigen-presenting cells are involved.66 The infiltrate of T cells mediates immunoreactivity against basal layer keratinocytes, which have altered surface antigens and adhesion molecules.67 LP is also linked to hepatitis C virus. Some individuals develop lichenoid lesions after exposure to drugs or film-processing chemicals. The age of onset is usually between 30 and 70 years. The disorder begins with flat purple, polygonal, pruritic, nonscaling papules 2 to 4 mm in size, usually located on the wrists, ankles, lower legs, and genitalia (Figure 46-11). New lesions are pale pink and evolve into a dark violet. Persistent lesions may be thickened and red, forming hypertrophic LP. Oral lesions (oral lichen planus) appear as lacy white rings that must be differentiated from leukoplakia or oral candidiasis and they may be precancerous lesions.68 Fine white lines, known as Wickham striae, can be seen throughout the oral lesions on magnification. These lesions also can develop on the penis and vulvovaginal area. More commonly, oral lesions do not ulcerate, but localized or extensive painful ulcerations do, and frequently, occur. Chronic ulcerated lesions become malignant in 1% of individuals with the disease. Thinning and splitting of nails are common, and part or all of the nail may be shed.
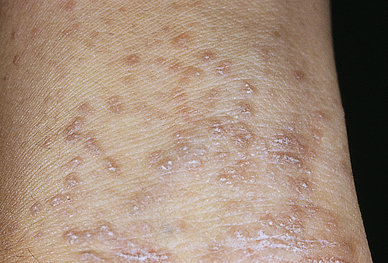
Pruritus is the most distressing symptom. The lesions are self-limiting and may last for months or years, with an average duration of 6 to 18 months. Postinflammatory hyperpigmentation is a common consequence of the lesion. Approximately 20% of individuals have a recurrence. Diagnosis is commonly made by the clinical appearance of the lesion; however, histopathologic diagnosis is recommended. For cutaneous LP, treatment is individualized and includes topical, intralesional, or systemic corticosteroids (second line for resistant LP), and systemic acitretin with/without adjuvant light therapy. Antihistamines are given for itching, and topical or systemic corticosteroids may be used to control inflammation. Short-term systemic glucocorticoids are indicated if oral lesions are severe or for LP involving three or more nails. Mucous membrane lesions are treated with potent topical steroids, topical retinoids, and systemic glucocorticoids.69–71
Acne Vulgaris
Acne vulgaris is an inflammatory disorder of the pilosebaceous follicle (the sebaceous gland contiguous with a hair follicle). It occurs most commonly during adolescence. Details of this disorder are presented in Chapter 47. Hidradenitis suppurativa (inverse acne) is summarized in What’s New? Hidradenitis Suppurativa (Inverse Acne).
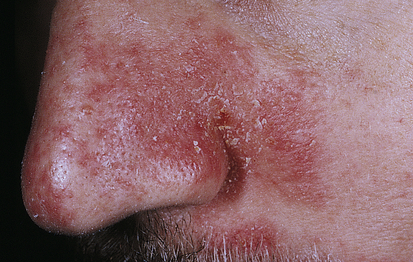
Acne Rosacea
Acne rosacea is a chronic, readily exacerbated, inflammatory skin disease with varied presentations and extent of involvement that develops primarily in middle-age adults. There are four types of lesions: erythematotelangiectatic, papulopustular, phymatous, and ocular.72 They occur in the middle third of the face, including the forehead, nose, cheeks, and chin (Figure 46-12). The cause is unknown, but immune-mediated inflammation may be a factor. The lesions are associated with chronic,
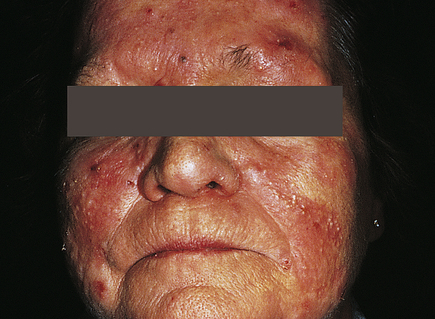
Pustules and erythema occur on the forehead, cheeks, and nose. (Courtesy Department of Dermatology, School of Medicine, University of Utah.)