Fig. 11.1
Stent for the femoral artery (Terumo, “MisagoTM”, Reprinted from [1] under special permission from Terumo Corporation)
The stents for the esophagus have a larger dimensions, 16–20 mm diameter, and are woven mesh tubes made of nitinol wires. Grafted stents, or covered stents, are also used for the esophagus.
Stents can be classified into two types depending on how they are deployed: balloon expandable and self-expandable. Balloon-expandable stents are most commonly used for coronary. Procedure of stenting is illustrated in Fig. 11.2. A doctor inserts a catheter through an artery from the groin, leg, or arm. X-ray angiography is used to know the location of the closure and the catheter. Contrast medium, a dye or other substances, is injected into a vein through the catheter near the area of blockage, so the blood flow through the arteries can be visualized on the monitors. The visibility of the stent under X-ray angiography is an important factor for the operation. After placing the stent, the vessel is checked with intravascular ultrasound (IVUS) or by optical coherence tomography (OCT). These devices can image the cross section of the stented vessel and are useful to check the status of the lesion area, e.g., cohesiveness of the stent to the vessel wall.
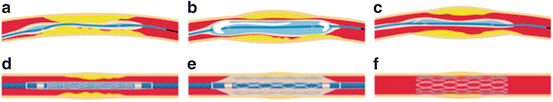

Fig. 11.2
Typical procedure for stenting. (a) Balloon catheter is inserted into the vessel to the site of the blockage. (b) Balloon is inflated to open the closed vessel. (c) Balloon is deflated and balloon catheter is retracted. (d) Catheter with a stent and balloon is inserted. (e) Balloon is inflated to open the stent. (f) Stent keeps the vessel open after the retraction of balloon and catheter
The design of stent strut varies largely depending on the producer as shown in Fig. 11.3. The complex shape of the stent is designed considering many factors, such as high hoop rigidity, ease of deployment, minimization of recoil, minimization of axial length change on deployment, uniform application of stress to the vessel wall, flexibility, conformability, and crossability. Stress concentration at both ends of the stent often causes restenosis.
Most of metallic stents are cut from a metallic tube by precision laser cutting. To minimize the internal stress introduced, annealing is applied to improve the fatigue life. In the case of self-expandable stent made of nitinol, shape-setting treatment is applied. Surface finishing of the stents is also very important. Various methods, such as mechanical polishing, blasting, horning, etching, and electropolishing, are used. Most of the stents have markers made of materials with high opacity for X-ray, e.g., platinum, gold, and tantalum, to ensure the visibility of stent position by X-ray angiography. These markers are welded to the struts near the ends of the stents. The possibility of galvanic corrosion has to be taken into consideration.
11.3 Balloon-Expandable Stents
Balloon-expandable stents are manufactured in the diameter that fits in the catheter sheath and expanded to the diameter of the blood vessel by inflating a balloon. Thus, the stents are plastically deformed. Figure 11.4 shows how the hoop stress in stents (lower part of the diagram) and in the vessel (upper part of the diagram) changes on deployment. As it is expanded from the initial diameter (4 mm in the figure), the stents experience the tensile hoop stress up to point b. As the stent touches the vessel of 7 mm diameter at point a, the vessel now experiences the tensile hoop stress which increases to point b. After deflating the balloon, the compressive hoop stress is applied to stents, and the stents recoil to the diameter of 8.25 mm, which corresponds to the diameter at which the stress on the blood vessel and that on the stent balance (point c).
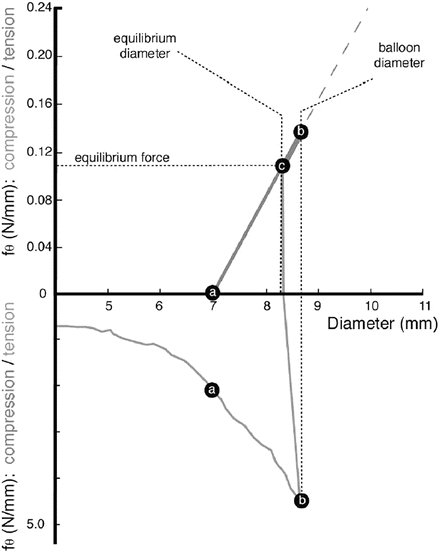

Fig. 11.4
Hoop stress of stent and vessel (Reprinted from [3], Copyright 2002, with permission from Elsevier)
A variety of metallic materials have been used for balloon-expandable stents as summarized in Table 11.1. Most widely used materials for balloon-expandable stents are 316L stainless steel and Co-Cr alloy. SUS316L is the most commonly used metallic material in the biomedical field. The formation of stable Cr2O3 passive film leads to better corrosion resistance. The tensile properties can be varied widely by processing conditions.
Table 11.1
Metallic materials used for stents
Material | SUS316L | Ta | Co-Cr | Ti | Pt-Cr steels | Nitinol |
|---|---|---|---|---|---|---|
Young’s modulus (GPa) | 192 | 61 | 200 | 100–120 | 191–203 | 50* |
Yield stress (MPa) | 175 | 200 | 550 | 310–490 | 460–480 | ~200 |
Tensile stress (MPa) | 480 | 500 | 720 | 380–640 | 824–834 | ~1,000 |
Tensile elongation (%) | >40 | >60 | 3 | 10–20 | 43.0–44.8 | ~10 |
Radiopacity | Normal | Very good | Good | Poor | Very good | Good |
In the early 2000s, there are a number of reports on the relation between the restenosis and strut thickness of a stent. Several clinical reports [4, 5] indicated that a reduced strut thickness lowers the restenosis rates as shown in Fig. 11.5. These reports drove the demands for stronger and more rigid materials. Co-Cr alloy has a higher strength and better radiopacity than 316L. It also has good corrosion resistance, has good fatigue resistance, and is nonmagnetic. The alloy has been used for medical implants since 1937. The first stent using Co-20Cr-35Ni-10Mo was developed by Medtronic as “DriverTM” in 2003. This alloy was originally developed as a heat-resistant alloy known as ElgiloyTM. The strut thickness was reduced to 80–90 μm compared to 130–140 μm of earlier SUS316L stents.
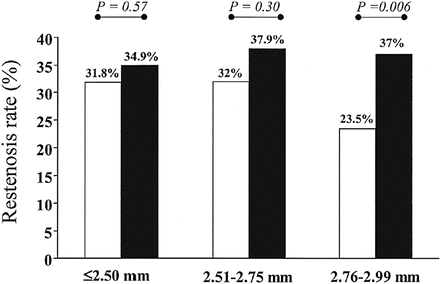

Fig. 11.5
Restenosis rates in lesions treated with a stent with a strut thickness of < 0.10 mm (thin group; open bars) and a stent with a strut thickness ≥ 0.10 mm (thick group; solid bars), according to the reference vessel diameter (≤2.50, 2.51–2.75 and 2.76–2.99 mm) (Reprinted from [4], Copyright 2002, with permission from Elsevier)
Strong demands for materials with a higher strength, rigidity, and radiopacity led to a new alloy specifically designed for coronary stents. Boston Scientific developed Pt-Cr steel, Fe – (32.5–33.5 wt%)Pt – (17.5–18.5 wt%)Cr – (2.43–2.83 wt%)Ni [6, 7]. To attain higher radiopacity, a part of Fe and Ni was substituted by Pt. The amount of Pt was designed to give a good balance between strength, ductility, and radiopacity. The alloy was used for OMEGATM stents which have a strut thickness of 81 μm and width of 91 μm.
11.4 Self-Expandable Stents
Self-expandable stents are manufactured to be the size of vessel diameter, or slightly larger, and are crimped and constrained in a sheath on a tip of catheter. When it is delivered to the intended site, the sheath is retracted and the stent deploys by itself. Most often, this self-deployment is achieved by superelasticity of shape memory alloy as shown in Fig. 11.6 [8]. Nitinol (TiNi alloy) is almost the only material used for self-expandable stents, due to its superior superelastic properties, good corrosion resistance, and biocompatibility. The basic aspect of nitinol and superelasticity can be found in Chap. 10 or elsewhere [9]. Superelasticity is a kind of pseudoelasticity induced by stress-induced martensitic transformation. As shown in Fig. 11.6, the stress-strain curve is characterized by a stress plateau and large hysteresis. A superelastic stent is in the parent phase when in an unconstrained condition and in the martensite phase when constrained, i.e., inside the sheath. A nitinol stent takes an advantage of the stress plateau and hysteresis. The stents can apply relatively gentle force corresponding to a lower stress plateau to the vessel wall for an extended range of strain. Meanwhile, when an external force is applied to deform or buckle the deployed stent, it resists with the higher stress corresponding to the upper plateau stress. This can be an advantage for the stent which often experiences severe bending or flattering, and thus, the self-expanding stents are most widely used in peripheral as well as biliary arteries. Biliary stents require maneuverability through loops, curvatures, and angulated anatomies and thus high flexibility. A self-expandable stent is also important for carotid and neurovascular vessels since a balloon-expandable stent cannot be used because of a high pressure required for the expansion, which may damage the thin wall of the brain vessels.
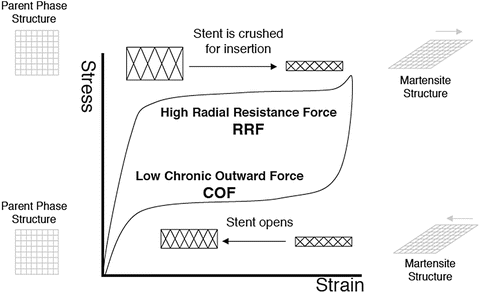

Fig. 11.6
The radial resistive force and chronic outward force as a function of the superelastic hysteresis loop (Reprinted from [8], Copyright 2004, with permission from Elsevier)
11.5 Drug-Eluting Stents (DES)
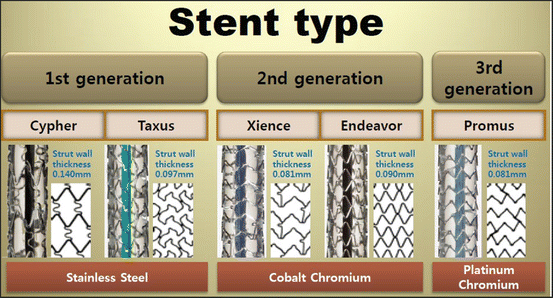
For bare-metal coronary stents, a clinical report indicated about 20 % of cases ended in restenosis and thrombosis that arise most typically ~30 days after stenting [10]. To minimize this reaction, most of the current coronary stents are drug-eluting stents (DES). The surface of a metallic stent is coated with non-degradable/degradable polymers containing an immunosuppressant or anticancer drug. The first-generation DESs were “Cypher” by Johnson & Johnson and “Taxus” by Boston Scientific. Both used 316L stent as a platform. Most of the second-generation DESs use Co-Cr (Abbott “Xience,” Medtronic “Resolute Integrity”) or Pt-Cr (Boston Scientific “Promus”) stents as the platform. The use of thin strut stents leads to early reendothelialization and is an advantage also for DES [10].
11.6 Bioabsorbable Stents
11.6.1 Emergence of the Research and Development for Bioabsorbable Stents
As described in the previous sections, metallic stents were incorporated into the cardiovascular intervention to give a mechanical support for vessel wall dissections, elastic recoil, and constrictive remodeling of the target lesion after the dilatation of a narrowed artery by a balloon catheter. However, there is still the problem of an in-stent restenosis by neointimal hyperplasia, which is an overgrowth of endothelial and/or smooth muscle cells. The causes of this overgrowth are considered as a physical damage of dilatation, a foreign-body reaction against a metallic stent, and a mechanical stimulation by the stent due to the difference in mechanical properties between the stent and the artery. To solve this problem pharmaceutically, a drug-eluting stent is introduced, which successfully reduced the ratio of in-stent restenosis as between 4 and 15 % [11], but it also caused late and very late stent thrombosis since the stent strut is not covered by the neointima which possibly causes strut malapposition. Therefore, patients should keep taking an antiplatelet drug for a long time such as over a year, which leads to a high risk of hemorrhage and precludes any kind of operation to treat other symptoms. This lack of endothelialization is not observed on the previous bare-metal stents and severely influences the patient’s remaining time and quality of life.
As the next generation of cardiovascular intervention, the research and development of bioabsorbable stents, or bioresorbable scaffolds, was started at the end of the twentieth century; the first clinical study of a biodegradable polymer stent was performed in Japan in 1998 [12]. After that, various attempts were made to satisfy the requirement for a bioabsorbable stent, which can dilate the narrowed artery; can support vascular dissections; can prevent elastic recoil, constructive remodeling, and neointimal overgrowth; and, finally, can disappear after a certain period of time, that is, when the physiological remodeling of the target lesion is achieved. The disappearance of the stent will be beneficial not only to avoid the prolonged inflammation at the implanted site causing neointimal hyperplasia but also to enable easy access to the downstream of the artery for another catheter intervention. Another benefit of bioabsorbable stents is the application to infants or children with rapid growth changing the size of organs and blood vessels.
As materials for bioabsorbable stents, not only biodegradable polymers such as poly-L-lactic acids and its copolymers but also metals such as magnesium alloys, iron-based alloys, and pure zinc are now under investigation. Some of them are under clinical studies (see Table 11.2), and no metallic stents but only two types of polymeric stents are available in the market [13]. In the rest of this section, the recent development of these metallic and polymeric stents will be briefly introduced.
Name | Material | Drug elution | Total strut thickness (μm)a | Stent coverage (%) | LLL at 6 months (mm) | TLR rate at 1 year (%) |
|---|---|---|---|---|---|---|
AMS®-1 | WE43 | − | 165 | 10 | 1.08 ± 0.49 (at 4 months) | 45 % |
DREAMS®1 | WE43 | + | 125 | 0.64 ± 0.50 | 9.1 % (at 6 months) | |
Igaki-Tamai® | PLLA | − | 170 | 24 | 0.91 ± 0.69 | |
Absorb®1.0
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|