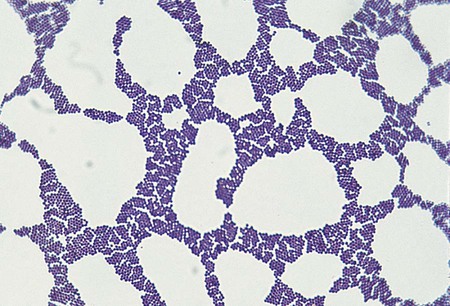
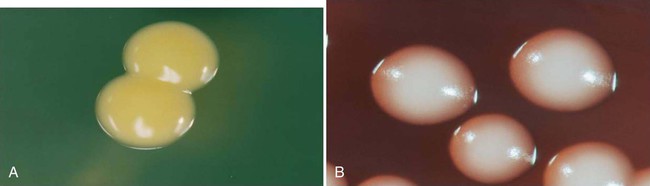
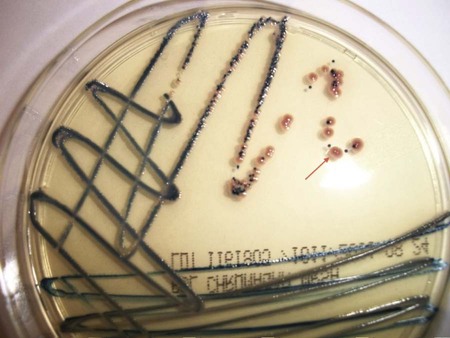
1. Describe the general characteristics of Staphylococcus spp. and Micrococcus spp., including oxygenation, microscopic gram staining characteristics, and macroscopic appearance on blood agar. 2. Describe the chemical principle of the media used for the isolation and differentiation of staphylococci, including 5% sheep blood agar, mannitol salt, phenyl-ethyl alcohol, and colistin nalidixic acid agars. 3. Explain the principle of the coagulase test, including the different principles associated with the slide versus the tube test and the clinical significance. 4. List the various types of diseases specifically associated with Micrococcus spp., S. aureus, S. saprophyticus, and S. epidermidis. 5. Outline the basic biochemical testing procedure to differentiate Staphylococcus spp. from Micrococcus spp., including coagulase negative and coagulase positive staphylococci. 6. Identify key biochemical reactions to identify the clinically significant Staphylococcus spp., and explain the chemical principle associated with each test. 7. Define methicillin-resistant staphylococcus aureus (MRSA) as it relates to antibiotic susceptibility. 8. Explain the D Zone test principle and clinical significance in the treatment of S. aureus. 9. Describe methods utilized to control the transmission of multiple drug resistant organisms such as MRSA within the community and health care settings. As outlined in Table 14-1, the staphylococci associated with infections in humans are colonizers of various skin and mucosal surfaces. There are three types of nasal carrier states associated with S. aureus: persistent carriers that harbor a single strain for an extended period of time, intermittent carriers that will harbor different strains over time, and then individuals that do not harbor any organisms or non-carriers. Because the carrier state is common among the human population, infections are frequently acquired when the colonizing strain gains entrance to a normally sterile site as a result of trauma or abrasion to the skin or mucosal surface. However, the traumatic event often may be so minor that it goes unnoticed. Health care workers have a high incidence of carrier state along with immunocompromised individuals, including those with insulin-dependent diabetes mellitus, long-term hemodialysis patients, and IV drug users. Vaginal carriage may be seen in premenopausal women. TABLE 14-1 Without question, S. aureus is the most virulent species of staphylococci encountered. A wide spectrum of factors, not all of which are completely understood, contribute to this organism’s ability to cause infections and disease. S. aureus and S. epidermidis produce a polysaccharide capsule that inhibits phagocytosis. The capsule, which is produced in various amounts by individual clinical isolates, may appear as a slime layer or biofilm, allowing the organisms to adhere to inorganic surfaces and circumventing the actions of antibiotics. The gram-positive cell wall chemical composition is also implicated in the mediation of pathogenesis. The peptidoglycan resembles the endotoxin effect of gram negatives by activating complement, interleukin 1 (IL-1), and acting as a chemotactic factor for the recruitment of PMNs. This cascade of events causes swelling and may lead to the exacerbation of tissue damage because of the additional virulent factors produced by the organisms. S. aureus produces a surface protein, known as protein A. This protein is bound to the cytoplasmic membrane of the organism and has a high affinity for the Fc receptor on IgG molecules as well as complement. This provides a mechanism for the organisms to bind the immune active molecules, decreasing the ability for clearance of the organism from the site of infection. Several toxins and enzymes mediate tissue invasion and survival at the infection site (Table 14-2). Cytotoxins alpha, beta, delta, and gamma are produced by a variety of species. Most strains of S. aureus produce alpha toxin, which disrupts the smooth muscle in blood vessels and is toxic to erythrocytes, leukocytes, hepatocytes, and platelets. Beta toxin, believed to work in conjunction with the alpha toxin, is a heat-labile sphingomyelinase, which catalyzes the hydrolysis of membrane phospholipids resulting in cell lysis. S. aureus, S. epidermidis, and S. haemolyticus have been identified as capable of producing Delta toxin, which is cytolytic to erythrocytes and demonstrates nonspecific membrane toxicity to other mammalian cells. Gamma toxin is produced by all strains of S. aureus and may actually function in association with the Panton-Valentine leukocidin (PVL). Elaboration of these factors is chiefly responsible for the various skin, wound, and deep tissue infections commonly caused by S. aureus. Many of these infections can rapidly become life threatening if not treated and managed appropriately. TABLE 14-2 Pathogenesis and Spectrum of Diseases The coagulase-negative staphylococci, among which S. epidermidis is the most commonly encountered, are substantially less virulent than S. aureus and are opportunistic pathogens. Their prevalence as nosocomial pathogens is as much, if not more, related to medical procedures and practices than to the organism’s capacity to establish an infection. Infections with S. epidermidis and, less commonly, S. haemolyticus and S. lugdunensis usually involve implantation of medical devices (see Table 14-2). This kind of medical intervention allows invasion by these normally noninvasive organisms. Two organism characteristics that do enhance the likelihood of infection include production of a slime layer or biofilm-facilitating attachment to implanted medical devices and the ability to acquire resistance to most of the antimicrobial agents used in hospital environments. S. lugdunensis infections resemble S. aureus infections. No special considerations are required for specimen collection and transport of the organisms discussed in this chapter. Refer to Table 5-1 for general information on specimen collection and transport. The majority of the genera included within this chapter produce spherical, gram-positive cells. However, some of the species within the Micrococcaceae or Dermacoccaceae exhibit rod-shaped cells and are motile. During cell division, the organisms divide along both longitudinal and horizontal planes, forming pairs, tetrads, and, ultimately, irregular clusters (Figure 14-1). Gram stains should be performed on young cultures, because very old cells may lose their ability to retain crystal violet and may appear gram variable or gram negative. Staphylococci appear as gram-positive cocci, usually in clusters. Micrococci typically appear as gram-positive cocci in tetrads, rather than large clusters. The additional related genera (i.e., Kytococcus, Nesterenkonia, Dermacoccus, Arthrobacter, and Kocuria) resemble the staphylococci microscopically. Selective media can also be used to isolate staphylococci from clinical material. Phenylethyl alcohol (PEA) or Columbia colistin-nalidixic acid (CNA) agars may be used to eliminate contamination by gram-negative organisms in heavily contaminated specimens such as feces. In addition, mannitol salt agar may be used for this purpose. This agar contains a high concentration of salt (10%), the sugar mannitol, and phenol red as the pH indicator. S. aureus ferments mannitol and produces a yellow halo on this media as a result of acid production altering the pH (Figure 14-2). CHROMagar (originally invented by Alain Rambach) is a selective and differential media for the identification of methicillin-resistant Staphylococcus aureus. The media are now available from a variety of manufacturers. These media are becoming more widely used for the direct detection of nasal colonization. The medium is selective because it contains cefoxitin, and MRSA is resistant to this antibiotic. The addition of chromogenic substrates hydrolyzed by the organisms produce a mauve-colored colony, allowing for the identification of the organisms. Other organisms will hydrolyze various chromogenic substances within the media, resulting in a variety of colored colonies from white to blue to green (Figure 14-3). Table 14-3 describes the colonial appearance and other distinguishing characteristics (e.g., hemolysis) of each genus and various staphylococcal species on 5% sheep blood agar. Growth on chocolate agar is similar. S. aureus yields colonies surrounded by a yellow halo on mannitol salt agar. In addition, small colony variants of S. aureus appear as small pinpoint, nonhemolytic and nonpigmented colonies on blood agar. Small colony variants (SCVs) may result from limited nutrients or other selective pressures and may revert to the normal S. aureus phenotype following subculture. However, other staphylococci (particularly S. saprophyticus) may also ferment mannitol and thus resemble S. aureus on this medium. TABLE 14-3 Colonial Appearance and Characteristics on 5% Sheep Blood Agar *Includes Kytococcus, Nesterenkonia, Dermacoccus, Kocuria, and Arthrobacter. The commercial systems for identification of Staphylococcus spp. and Micrococcus spp. are discussed in Chapter 13. Most commercial systems are successful in the identification of S. aureus, S epidermidis, and S. saprophyticus. The identification of the other species varies from system to system. In addition, automated systems may not correctly identify nutritionally variant forms such as small colony variants and other unusual isolates. Gram stains are used in the clinical laboratory as the initial presumptive identification method for all gram-positive cocci. Microscopic along with macroscopic colonial morphology (see Table 14-3) provides a presumptive identification. The Staphylococci spp. and Micrococci spp. are distinguishable from the related family Streptococcaceae (see Chapter 15) by the catalase test. Table 14-4 shows how the catalase-positive, gram-positive cocci can be differentiated. Because they may show a pseudocatalase reaction—that is, they may appear to be catalase-positive—Aerococcus and Enterococcus are included in Table 14-4; Rothia (formerly Stomatococcus) is included for the same reason. Once an organism has been characterized as a gram-positive, catalase-positive, coccoid bacterium, complete identification may involve a series of tests, including (1) atmospheric requirements, (2) resistance to 0.04 U of bacitracin (Taxo A disk) and furazolidone, and (3) possession of cytochrome C as determined by the microdase (modified oxidase) test. However, in the busy setting of many clinical laboratories, microbiologists proceed immediately to a coagulase test based on recognition of a staphylococcal-like colony and a positive catalase test. TABLE 14-4 Differentiation among Gram-Positive, Catalase-Positive Cocci
Staphylococcus, Micrococcus, and Similar Organisms
Epidemiology
Organism
Habitat (Reservoir)
Mode of Transmission
Staphylococcus aureus
Normal flora:
Anterior nares
Nasopharynx
Perineal area
Skin
Colonizer of mucosa
Endogenous strain: sterile site by traumatic introduction (e.g., surgical wound or microabrasions)
Direct contact: person-to-person, fomites
Indirect contact: aerosolized
Staphylococcus epidermidis
Normal flora:
Skin
Mucous membranes
Endogenous strain: sterile site, by implantation of medical devices (e.g., shunts, prosthetic devices)
Direct contact: person-to-person
Staphylococcus haemolyticus
Staphylococcus lugdunensis
Normal flora:
Skin
Mucous membranes (low numbers)
Same as previously indicated for S. epidermidis
Staphylococcus saprophyticus
Normal flora:
Skin
Genitourinary tract
Mucosa
Endogenous strain: sterile urinary tract, notably in young, sexually active females
Micrococcus spp.
Kocuria spp.
Kytococcus spp.
Normal flora:
Skin
Mucosa
Oropharynx
Endogenous strain: uncertain
Rarely implicated in infections
Immunocompromised hosts: brain abscess, meningitis, pneumonia, endocarditis
Pathogenesis and Spectrum of Disease
Organism
Virulence Factors
Spectrum of Diseases and Infections
Staphylococcus aureus
Polysaccharide capsule: Inhibits
phagocytosis (slime layer or biofilm)
Peptidoglycan: activates complement, IL-1, chemotactic to PMNs
Teichoic acids: species specific, mediate binding to fibronectin
Protein A: affinity for Fc receptor of IgG and complement.
Exotoxins:
Cytotoxins (alpha, beta, delta and gamma)
Leukocidins, PVL
Exfoliative toxins
Enterotoxins: A-E, G-I heat stable
Toxic Shock Syndrome Toxin I (TSST-1); pyrogenic exotoxin C
Enzymes:
Coagulase, clumping factor
Catalase
Hyaluronidase
Fibrinolysin: staphylokinase
Lipases
Nucleases
Penicillinase
Carriers: Persistent in older children and adults, nasopharynx
Toxin mediated:
Scalded skin syndrome: Ritter’s disease involves ≥90% of the body, pemphigus neonatorum is the localized form evident by a few blisters; both are exfoliative dermatitis caused by toxins A and B
Toxic shock syndrome
Food poisoning; preformed enterotoxins, resulting in gastrointestinal symptoms within 2-6 hours of consumption of contaminated food
Localized skin infections: folliculitis
Furuncles and carbuncles
Impetigo
Tissue and systemic:
Wounds
Bacteremia; any localized infection can become invasive and lead to bacteremia
Endocarditis
Osteomyelitis
Cerebritis
Pyelonephritis
Staphylococcus epidermidis
Exopolysaccharide “slime” or biofilm; antiphagocytic.
Exotoxins: delta toxin
Normal flora: nosocomial Infections: bacteremia associated with indwelling vascular catheters; endocarditis involving prosthetic cardiac valves (rarely involves native valves); infection at intravascular catheter sites, frequently leading to bacteremia; and other infections associated with CSF shunts, prosthetic joints, vascular grafts, postsurgical ocular infections, and bacteremia in neonates under intensive care
S. haemolyticus and S. lugdunensis
Uncertain; probably similar to those described for S. epidermidis
S. haemolyticus
Endocarditis
Bacteremia
Peritonitis
Urinary tract
Wound, bone, and joint infections
S. lugdunensis
Bacteremia
Wound infections
Endocarditis
Endophthalmitis
Septic arthritis
Vascular catheter infections
Urinary tract infections
S. saprophyticus
Uncertain
Urinary tract infections in sexually active, young females; infections in sites outside urinary tract are not common
S. schleiferi
Uncertain
Endocarditis
Septicemia
Osteomyelitis
Joint infections
Wounds
Micrococcus spp.,
Kocuria spp.
Kytococcus spp.
Unknown; probably of extremely low virulence
Usually considered contaminants of clinical specimens; rarely implicated as cause of infections in humans
Laboratory Diagnosis
Specimen Collection and Transport
Direct Detection Methods
Microscopy
Cultivation
Media of Choice
Colonial Appearance
Organism
Appearance
Micrococcus spp. and related organisms*
Small to medium (1-2 µm); opaque, convex; nonhemolytic; wide variety of pigments (white, tan, yellow, orange, pink)
Staphylococcus aureus
Medium to large (0.5-1.5 µm); smooth, entire, slightly raised, low convex, opaque; most colonies pigmented creamy yellow; most colonies beta-hemolytic
S. epidermidis
Small to medium; opaque, gray-white colonies; most colonies nonhemolytic; slime-producing strains are extremely sticky and adhere to the agar surface
S. haemolyticus
Medium; smooth, butyrous, and opaque; beta-hemolytic
S. hominis
Medium to large; smooth, butyrous, and opaque; may be unpigmented or cream-yellow-orange
S. lugdunensis
Medium to large; smooth, glossy, entire edge with slightly domed center; unpigmented or cream to yellow-orange, may be β-hemolytic
S. warneri
Resembles S. lugdunensis
S. saprophyticus
Large; entire, very glossy, smooth, opaque, butyrous, convex; usually white but colonies can be yellow or orange
S. schleiferi
Medium to large; smooth, glossy, slightly convex with entire edges; unpigmented
S. intermedius
Large; slightly convex, entire, smooth, glossy, translucent; usually nonpigmented
S. hyicus
Large; slightly convex, entire, smooth, glossy, opaque; usually nonpigmented
S. capitis
Small to medium; smooth, slightly convex, glistening, entire, opaque; S. capitis subsp. urealyticus usually pigmented (yellow or yellow-orange); S. capitis subsp. capitis is nonpigmented
S. cohnii
Medium to large; convex, entire, circular, smooth, glistening, opaque; S. cohnii subsp. urealyticum usually pigmented (yellow or yellow-orange); S. cohnii subsp. cohnii is nonpigmented
S. simulans
Large; raised, circular, nonpigmented, entire, smooth, slightly glistening
S. auricularis
Small to medium; smooth, butyrous, convex, opaque, entire, slightly glistening; nonpigmented
S. xylosus
Large; raised to slightly convex, circular, smooth to rough, opaque, dull to glistening; some colonies pigmented yellow or yellow-orange
S. sciuri
Medium to large; raised, smooth, glistening, circular, opaque; most strains pigmented yellow in center of colonies
S. caprae
Small to medium; circular, entire, convex, opaque, glistening; nonpigmented
Approach to Identification
Resistance to:
Organism
Catalase
Microdase (modified oxidase)
Aerotolerance
Bacitracin (0.04 U)a
Furazolidone (100 µg)a
Lysostaphin (200 µg/µL)
Staphylococcus
+b
−c
FA
R
S
S
Micrococcus (and related organisms)
+
+
Ad
S
R
Re
Macrococcus
+
+
±
+
S
S
Rothia
±
−
FA
R or S
R or S
R
Aerococcus
−f
−
FAg
S
S
R
Alloiococcus
±
−
A
ND
ND
ND
Enterococcus
−f
−
FA
R
S
R
Streptococcus
−
−
FA
+d
−
+ ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Staphylococcus, Micrococcus, and Similar Organisms