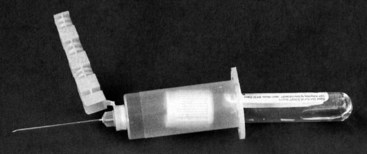
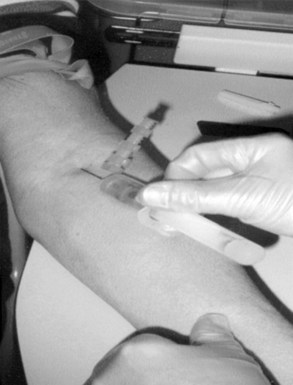
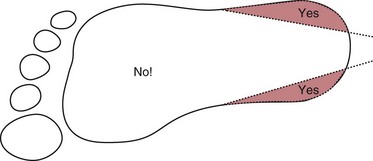
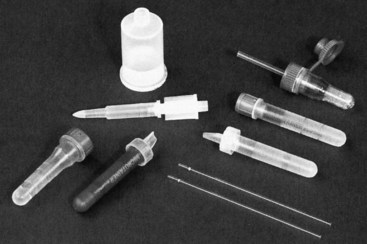
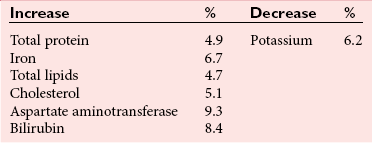
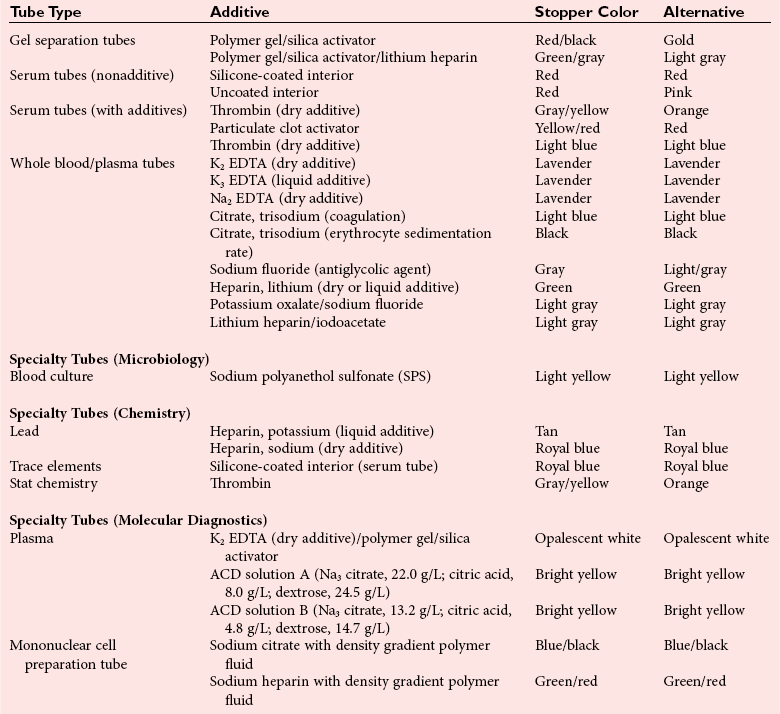
Chapter 7 Types of biological specimens that are analyzed in clinical laboratories include (1) whole blood; (2) serum; (3) plasma; (4) urine; (5) feces; (6) saliva; (7) spinal, synovial, amniotic, pleural, pericardial, and ascitic fluids; and (8) various types of solid tissue. The Clinical and Laboratory Standards Institute (CLSI) has published several procedures for collecting many of these specimens under standardized conditions.4–16 In the clinical laboratory, venipuncture is defined as all of the steps involved in obtaining an appropriate and identified blood specimen from a patient’s vein.12 Before any specimen is collected, the phlebotomist must confirm the identity of the patient.4 Two or three items of identification should be used (e.g., [1] name, [2] medical record number, [3] date of birth, [4] address if the patient is an outpatient). In specialized situations, such as paternity testing or other tests of medico-legal importance, establishment of a chain of custody for the specimen may require additional patient identification, such as a photograph, provided as part of the identification process or taken to confirm the identity of the patient. Before collection of a specimen, a phlebotomist should dress in personal protective equipment (PPE), such as an impervious gown and gloves applied immediately before approaching the patient, to adhere to standard precautions against potentially infectious material and to limit the spread of infectious disease from one patient to another.14 If the phlebotomist is to collect a specimen from a patient in isolation in a hospital, the phlebotomist must put on a clean gown and gloves and a face mask and goggles before entering the patient’s room. The face mask limits the spread of potentially infectious droplets, and the goggles limit the possible entry of infectious material into the eye. The extent of the precautions required will vary with the nature of the patient’s illness and the institution’s policies and bloodborne pathogen plan, to which a phlebotomist must adhere. If airborne precautions are indicated, the phlebotomist must wear an N95 TB respirator. Before performing a venipuncture, the phlebotomist should estimate the volume of blood to be drawn and should select the appropriate number and types of tubes for the blood, plasma, or serum tests requested. In many settings, this will be facilitated by computer-generated collection recommendations and should be designed to collect the minimum amount necessary for testing. The sections below on “Order of Draw for Multiple Collections” and “Collection With Evacuated Blood Tubes” discuss in greater detail the recommended order of draw for multiple specimens and types of tubes. In addition to tubes, an appropriate needle must be selected. The most commonly used sizes are 19 to 22 gauge. (The larger the gauge number, the smaller the bore.) The usual choice for an adult with normal veins is 20 gauge; if veins tend to collapse easily, a size 21 is preferred. For volumes of blood from 30 to 50 mL, an 18-gauge needle may be required to ensure adequate blood flow. A needle is typically 1.5 inches (3.7 cm) long, but 1-inch (2.5-cm) needles, usually attached to a winged or butterfly collection set, are also used. All needles must be sterile, sharp, and without barbs. If blood is drawn for trace element measurements, the needle should be stainless steel and should be known to be free from contamination. The median cubital vein in the antecubital fossa, or crook of the elbow, is the preferred site for collecting venous blood in adults because the vein is large and is close to the surface of the skin.12,20 Veins on the back of the hand or at the ankle may be used, although these are less desirable and should be avoided in people with diabetes and other individuals with poor circulation. In the inpatient setting, it is appropriate to collect blood through a cannula that is inserted for long-term fluid infusions at the time of first insertion to avoid the need for a second stick. For severely ill individuals and those requiring many intravenous injections, an alternative blood-drawing site should be chosen. Selection of a vein for puncture is facilitated by palpation. An arm containing a cannula or an arteriovenous fistula should not be used without consent of the patient’s physician. If fluid is being infused intravenously into a limb, the fluid should be shut off for 3 minutes before a specimen is obtained and a suitable note made in the patient’s chart and on the result report form. Specimens obtained from the opposite arm are preferred.12 Specimens below the infusion site in the same arm may be satisfactory for most tests, except for those analytes that are contained in the infused solution (e.g., glucose, electrolytes). The time at which a specimen is obtained is important for those blood constituents that undergo marked diurnal variation (e.g., corticosteroids, iron) and for those used to monitor drug therapy (see Chapter 34). For most current molecular diagnostic tests, the time of day is unlikely to contribute to altered or invalid test results. Furthermore, timing is important in relation to specimens for alcohol or drug measurements in association with medico-legal considerations. After the skin is cleaned, a blood pressure cuff or a tourniquet is applied 4 to 6 inches (10 to 15 cm) above the intended puncture site (distance for adults). This obstructs the return of venous blood to the heart and distends the veins (venous occlusion). When a blood pressure cuff is used as a tourniquet, it is usually inflated to approximately 60 mm Hg (8.0 kPa). Tourniquets typically are made from precut soft rubber strips or from Velcro. It is rarely necessary to leave a tourniquet in place for longer than 1 minute, but even within this short time the composition of blood changes. Although the changes that occur in 1 minute are slight, marked changes have been observed after 3 minutes for many chemistry analytes (Table 7-1). No known changes affect molecular diagnostics. TABLE 7-1 Changes in Composition of Serum When Venous Occlusion Is Prolonged from 1 Minute to 3 Minutes*† *To estimate the probable effect of a factor on results, relate percent increase or decrease shown (or intimated) in table to analytical variation (±% CV) routinely found for analytes. †Mean values obtained from 11 healthy individuals. From Statland BE, Bokelund H, Winkel P. Factors contributing to intraindividual variation of serum constituents: effects of posture and tourniquet application on variation of serum constituents in healthy subjects. Clin Chem 1974;20:1513-9. The composition of blood drawn first—that is, the blood closest to the tourniquet—is most representative of the composition of circulating blood. The first-drawn specimen should therefore be used for those analytes such as calcium that are pertinent to critical medical decisions.25 Blood drawn later shows a greater effect from venous stasis. Thus the first tube may show a 5% increase in protein, whereas the third tube may show a 10% change.22 The concentration of protein-bound constituents is also influenced by stasis. Prolonged stasis may increase the concentration of protein or protein-bound constituents by as much as 15%. A uniform procedure for the order of draw for tests should therefore be established (see later). If it is possible to collect only a small volume of blood, the priority of which tests to perform should be established. Pumping of the fist before venipuncture should be avoided because it causes an increase in plasma potassium, phosphate, and lactate concentrations. Lowering of blood pH by accumulation of lactate causes the plasma ionized calcium concentration to increase.24 The ionized calcium concentration reverts to normal 10 minutes after the tourniquet is released. In a few patients, backflow from blood tubes into veins occurs owing to a decrease in venous pressure. The dangerous consequences of this occurrence may be prevented if only sterile tubes are used for collection of blood. Backflow is minimized if the arm is held downward and blood is kept from contact with the stopper during the collection procedure. To minimize problems if backflow should occur, and to optimize the quality of specimens—especially to prevent cross-contamination with anticoagulants—blood should be collected into tubes in the order outlined in Table 7-2. This table also provides the recommended number of inversions for each tube type because it is critical that complete mixing of any additive with the blood collected be accomplished as quickly as possible. TABLE 7-2 Recommended Order of Draw for Multiple Specimen Collection Modified from information in CLSI. Tubes and additives for venous blood specimen collection: CLSI-approved standard H1-A6, 6th edition. Wayne, Pa: Clinical and Laboratory Standards Institute, 2010; Kiechle FL, ed. So you’re going to collect a blood specimen: an introduction to phlebotomy, 11th edition. Northfield, Ill: College of American Pathologists, 2005. Evacuated blood tubes are usually considered to be less expensive and are more convenient and easier to use than syringes, and thus are the collection device of choice in many institutions. Evacuated blood tubes may be made of soda-lime or borosilicate glass or plastic (polyethylene terephthalate). Because of the decreased likelihood of breakage and subsequent exposure to infectious materials, many laboratories have converted from glass tubes to plastic tubes. Several types of evacuated tubes may be used for venipuncture collection.12 They vary by the type of additive added and the volume of the tube. The different types of additives are identified by the color of the stopper used (Table 7-3). Serum or plasma separator tubes are available that contain an inert, thixotropic, polymer gel material with a specific gravity of approximately 1.04. Aspiration of blood into the tube and subsequent centrifugation displace the gel, which settles like a disk between cells and supernatant when the tube is centrifuged. A minimum relative centrifugal force (RCF) of 1100 ×g is required for gel release and barrier formation in most tubes. Release of intracellular components into the supernatant is prevented by the barrier for several hours or, in some cases, for a few days. These separator tubes may be used as primary containers from which serum or plasma can be directly aspirated by a number of analytical instruments. Additional tubes, not listed, are sold for special applications, such as RNA isolation. These less common tubes must be validated by each laboratory before use if not approved by the manufacturer for the specific analysis to be conducted. TABLE 7-3 Coding of Stopper Color to Indicate Additive in Evacuated Blood Tube Modified from information in CLSI. Tubes and additives for venous blood specimen collection: CLSI-approved standard H1-A6, 6th edition. Wayne, Pa: Clinical and Laboratory Standards Institute, 2010; Becton Dickinson Web page (http:/www.bd.com/). Stoppers may contain zinc, invalidating the use of evacuated blood tubes for zinc measurement, and TBEP [tris(2-butoxyethyl) phosphate], a constituent of rubber, which may interfere with the measurement of certain drugs. With time, the vacuum in evacuated tubes is lost and their effective draw diminishes. The silicone coating also decays with age. Therefore the stock of these tubes should be rotated and careful attention paid to the expiration date. Blood collected into a tube containing one additive should never be transferred into other tubes, because the first additive may interfere with tests for which a different additive is specified. Additionally, transfer of the additive from one tube to another should be minimized (or adverse effects reduced) through strict adherence to recommendations for order of tube use (see Table 7-2). A typical system for collecting blood in evacuated tubes is shown in Figure 7-1.17 This is an example of a commonly used single-use device that incorporates a cover that is designed to be placed over the needle when collection of the blood is complete, thereby reducing the risk of puncture of the phlebotomist by the now contaminated needle. A needle or winged (butterfly) set is screwed into the collection tube holder, and the tube is then gently inserted into this holder. The tube should be gently tapped to dislodge any additive from the stopper before the needle is inserted into a vein; this prevents aspiration of the additive into the patient’s vein. After the skin has been cleaned, the needle should be guided gently into the patient’s vein (Figure 7-2); once the needle is in place, the tube should be pressed forward into the holder to puncture the stopper and release the vacuum. As soon as blood begins to flow into the tube, the tourniquet should be released without moving the needle (see earlier discussion on venous occlusion). The tube is filled until the vacuum is exhausted. It is critically important that the evacuated tube be filled completely. Many additives are provided in the tube based on a “full” collection; deviation or short draws can be a source of preanalytical error because they can significantly affect test results.7 Once the tube is filled completely, it should be withdrawn from the holder, mixed gently by inversion, and replaced by another tube, if this is necessary. Other tubes may be filled using the same technique with the holder in place. When several tubes are required from a single blood collection, a shut-off valve—consisting of rubber tubing that slides over the needle opening—is used to prevent spillage of blood during exchange of tubes. When blood collection is complete and the needle withdrawn, the patient should be instructed to hold a dry gauze pad over the puncture site, with the arm raised to lessen the likelihood of leakage of blood. The pad may then be held in place by a bandage or by a nonadhesive strap (which avoids pulling hairs on the arm when it is removed); these are removed after 15 minutes. With a collection device, such as that shown in Figure 7-1, the needle is covered, and the needle and the tube holder are immediately discarded into a sharps container. In the event that a winged (butterfly) set is used, the wings are pushed forward to cover the needle, or with newer available equipment, a button is pressed, releasing a spring that retracts the needle. If a syringe was used, the needle and syringe (still attached) should be discarded in a hazardous waste receptacle. Skin puncture is an open collection technique in which the skin is punctured by a lancet and a small volume of blood is collected into a microdevice. Skin puncture blood is more like arterial blood than venous blood. In practice, it is used in situations in which (1) sample volume is limited (e.g., pediatric applications), (2) repeated venipunctures have resulted in severe vein damage, or (3) patients have been burned or bandaged and veins therefore are unavailable for venipuncture. This technique is also commonly used when the sample is to be applied directly to a testing device in a point-of-care testing situation or to filter paper. It is most often performed on (1) the tip of a finger, (2) an earlobe, and (3) the heel or big toe of infants. For example, in an infant younger than 1 year, the lateral or medial plantar surface of the foot should be used for skin puncture; suitable areas are illustrated in Figure 7-3.1 In older children, the plantar surface of the big toe may also be used, although blood collection from anywhere on the foot should be avoided on ambulatory patients. The complete procedure for collecting blood from infants using skin puncture is described in a CLSI document.10 To collect a blood specimen by skin puncture, the phlebotomist first thoroughly cleans the skin with a gauze pad saturated with an approved cleaning solution, as outlined earlier for venipuncture. If an alcohol swab is used, the alcohol must be allowed to evaporate from the skin so that hemolysis does not occur. When the skin is dry, it is quickly punctured by a sharp stab with a lancet. The depth of the incision should be less than 2.5 mm to prevent contact with bone. To minimize the possibility of infection, a different site should be selected for each puncture. The finger should be held in such a way that gravity assists collection of blood at the fingertip and the lancet held to make the incision as close to perpendicular to the fingernail as possible.20 Massage of the finger to stimulate blood flow should be avoided because it causes the outflow of debris and tissue fluid, which does not have the same composition as plasma. To improve circulation of the blood, the finger (or the heel in the case of heelsticks) may be warmed by application of a warm, wet washcloth or a specialized device, such as a heel warmer, for 3 minutes before the lancet is applied. The first drop of blood is wiped off, and subsequent drops are transferred to the appropriate collection tube by gentle contact. Filling should be done rapidly to prevent clotting, and introduction of air bubbles should be prevented. As the name suggests, blood is collected into capillary blood tubes by capillary action. A variety of collection tubes are commercially available (Figure 7-4). Containers are commercially available that contain different anticoagulants, such as sodium and ammonium heparin, and some are available in brown glass for collection of light-sensitive analytes, such as bilirubin (see later section on anticoagulants). As with evacuated blood tubes, to prevent the possibility of breakage and the spread of infection, capillary devices frequently are plastic or coated with plastic. A disadvantage of some of the collection devices shown in Figure 7-4 is that blood tends to pool in the mouth of the tube and must be flicked down the tube, creating a risk of hemolysis. Drop-by-drop collection should be avoided because it increases hemolysis. The correct order of filling of these devices is the same as for evacuated blood tubes (see Table 7-2). For collection of blood specimens on filter paper for molecular genetic testing and neonatal screening,5 the skin is cleaned and punctured as described previously. The first drop of blood should be wiped away. Then the filter paper is gently touched against a large drop of blood that is allowed to soak into the paper to fill the marked circle. Only a single application per circle should be made to prevent nonuniform analyte concentration.5 The paper is examined to verify that there has been complete penetration of the paper. The procedure is repeated to fill all the circles. Avoid milking or squeezing the finger or foot because this procedure contributes tissue fluids. The filter papers should be air-dried (generally for 2 to 3 hours to prevent mold or bacterial overgrowth) before storage in a properly labeled paper envelope. Blood should never be transferred onto filter paper after it has been collected in capillary tubes because partial clotting may have occurred, compromising the quality of the specimen. However, blood collected into an evacuated tube containing an anticoagulant may be applied directly to the filter paper. This is a convenient way to store a sample for possible future molecular testing (with patient consent). These blood spots are handled in the same manner as neonatal screening specimens, with air drying and storage in a dry protected environment. Arterial puncture requires considerable skill and is usually performed only by physicians or specially trained technicians or nurses. Preferred sites of arterial puncture are, in order, the (1) radial artery at the wrist, (2) brachial artery in the elbow, and (3) femoral artery in the groin. Because leakage of blood from the femoral artery tends to be greater, especially in the elderly, sites in the arm are used most often. The proper technique for arterial puncture is described in a CLSI document.11
Specimen Collection and Processing
Types of Specimens
Blood
Venipuncture
Preliminary Steps
Location
Timing
Venous Occlusion
Order of Draw for Multiple Blood Specimens
Stopper Color
Contents
Inversions
Yellow
Sterile media for blood culture
8
Royal blue
No additive
0
Clear
Nonadditive; discard tube if no royal blue used
0
Light blue
Sodium citrate
3-4
Gold/red
Serum separator tube
5
Red/red, orange/yellow, royal blue
Serum tube, with or without clot activator, with or without gel
5
Green
Heparin tube with or without gel
8
Tan (glass)
Sodium heparin
8
Royal blue
Sodium heparin, sodium EDTA
8
Lavender, pearl white, pink/pink, tan (plastic)
EDTA tubes, with or without gel
8
Gray
Glycolytic inhibitor
8
Yellow (glass)
ACD for molecular studies and cell culture
8
Collection With Evacuated Blood Tubes
Completion of Collection
Skin Puncture
Arterial Puncture
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Specimen Collection and Processing