Chapter 4 Barbara Resnick and Marilyn Winterton Edmunds Over the past century, the number of older adults has increased more than 10-fold and continues to increase. In the year 2000, approximately 35 million people were aged 65 or older (National Center for Health Statistics, 2007). Women who reach age 65 can expect to live an additional 19 years, and men at age 65 can expect to live an additional 16 years. Because the length of life has increased, older adults are living longer with chronic illnesses (Ray & Street, 2005). Specifically, nearly 60% of older African American adults have high blood pressure, and a growing share of elderly African Americans, Hispanics, and Native Americans have diabetes. Older adults make up 14% of the population yet account for 30% of medication expenditures. Up to 82% are taking at least one drug (Frankfort et al, 2006). Among a national sample of 2950 older adults, 12% took 10 medications daily and 23% were taking 5 prescription medications (Frankfort et al, 2006). The most common drug classes used are cardiovascular, analgesic, and central nervous system drugs (Passarelli et al, 2005). In addition, these individuals may add supplements, vitamins, and various home remedies to the regimen. Research has suggested that it is the number of pills a patient takes every day rather than the number of medications taken that is correlated with adverse drug reactions (ADRs) (Cramer, 2002). Because of a variety of issues, including limited knowledge of the impact of specific drugs on older adults, multiple medication use, and normal age- and disease-associated changes, older adults are at increased risk of experiencing ADRs (Bourne, 2007). It is essential for the health care provider to consider these issues before prescribing medications for this population. A 2011 Centers for Disease Control and Prevention (CDC) study in the New England Journal of Medicine indicated that warfarin, insulin, oral antiplatelet drugs, and oral hypoglycemic agents are responsible for more than two thirds of emergency hospital admissions among people aged 65 and older. The researchers indicated that better management of antithrombotic and antidiabetic agents can lead to better care for seniors (Budnitz et al, 2011). Functional assessment is the core concept of geriatrics. When one is evaluating elderly patients, it is important to assess their ability to function in addition to what medical illnesses they may have. Classic assessment tools include Katz’s Activities of Daily Living (ADLs) to assess mobility and the Folstein Mini-Mental Status Examination (MMSE) to assess cognitive function. Once medications have been started, it is essential to evaluate for the efficacy of drugs and for ADRs. Evaluation also must include any changes in the functional status of the patient that may result from medications. Common expressions of functional decline include weight loss, decline in mobility, incontinence, and falls (Gallo et al, 2006). The overall significance of changes in the absorption of drugs with aging is not completely clear. There appears to be little, if any, significance in the amount of drug absorbed when drugs are passively absorbed. However, some reduced absorption has been noted in older adults for some compounds that are actively absorbed, such as galactose, calcium, thiamine, and iron (Bourne, 2007). Physiologic changes that affect the GI tract include a reduction in acid output and the subsequent alkaline environment. These changes affect drugs such as B12 that require an acid medium for absorption, although this has not been well documented. Reductions in blood flow, enzyme activity, gastric emptying, and bowel motility may support the delay in absorption of some drugs, although such reductions probably have minimum, if any, effect on the extent of absorption. Further support for the insignificance of these physiologic changes is based on the fact that most drugs are absorbed via passive diffusion. Because the GI tract has such a large surface area, the extent of absorption of most drugs is not affected. Nonionized forms of a drug (i.e., those that are more lipid soluble) are more readily absorbed than ionized forms. If a drug such as an antacid is administered, the GI pH will be raised and the absorption of acidic drugs may be delayed or decreased. Compounds such as iron or calcium and certain vitamins that depend on active transport mechanisms and thus the delivery of oxygen for absorption may be affected by decreased blood flow in the aging patient’s GI tract (Brunton, Chabner, & Knollman, 2010). The distribution of drugs in the body, which is dependent on the chemical composition of the agent involved, may be affected by the aging process. As individuals age, the decline in total body water and lean body mass may affect drugs that are distributed into these areas (Box 4-1). Changes in total body water and lean body mass may result in limited distribution of drugs into these areas. Unadjusted dosing can result in increased serum concentrations, leading to an enhanced effect or toxicity. Specifically, as we age, decreased lean body mass usually is coupled with an increase in total body fat. Changes in body fat are reported as increasing from 18% to 36% in males and from 33% to 48% in females from the ages of 15 to 60 years. The increase in body fat can result in an increase in the volume of distribution of lipid-soluble drugs, leading to drug accumulation and the potential for toxicity. For example, certain drugs such as diazepam and chlordiazepoxide have a higher volume of distribution than do other anxiolytics, such as lorazepam and oxazepam, because of the lipid solubility of the former two drugs. Box 4-2 lists additional examples of lipid-soluble drugs that may require downward adjustments in dose and slowly titrated increases if used in the elderly. The risk of accumulation and toxicity is a real concern with high doses of these agents in the elderly (Bigos et al, 2006; Brunton et al, 2010). Another common age change that affects the distribution of drugs in older adults is a decrease in serum albumin. Albumin concentrations decrease slightly with age in most elderly patients, although significant changes that may affect drug therapy may be seen in the chronically ill or in malnourished elderly patients (Bourne, 2007). Albumin is the most common protein that binds to various acidic drugs. Significant decreases in albumin may result in a greater free concentration of highly protein-bound drugs. Box 4-3 lists some drugs that have significant protein binding, which may result in increased free concentrations when albumin is significantly reduced. Generally, drugs that are highly protein bound to albumin should be prescribed in reduced doses for patients with low serum albumin values (Bourne, 2007). A practical example of the clinical significance of this relationship can be described with the anticonvulsant phenytoin. In an elderly patient with a low serum albumin concentration (normal, 3.5 to 5 mg/dl), the phenytoin level reported from the laboratory will reflect both the bound and free concentrations and, in a hypoalbuminemic individual, may appear normal or even subtherapeutic. This occurs because of the greater free amounts of phenytoin that are getting into tissue and acting at the receptor level but are not portrayed in the total serum level. The actual level may be much higher or even in the toxic range. Treatment decisions for older adults should not be based solely on drug levels but must be reached after consideration of both patient characteristics and anticipated drug levels. Other protein changes may also have an influence on drug therapy. Patients with acute disease, such as a myocardial infarction, respiratory distress, or infection, for example, may experience increases in α-1-glycoprotein, an acute phase reactant protein. This may result in the increased binding of weakly basic drugs, including propranolol or lidocaine, and a less-than-normal response to therapy. Data on the real significance of changes in α1-glycoprotein and drug therapy in the elderly are lacking (Bigos et al, 2006; Brunton et al, 2010). The significance of hepatic blood flow changes may be seen with drugs that have a high first-pass metabolism or a high extraction ratio in the liver. These drugs are considered to have flow-limiting metabolism. When flow is reduced, as may occur with aging, less drug is metabolized and increased amounts may be present in active form in the blood. When drugs with first-pass metabolism are prescribed for the elderly, lower doses may be necessary. Examples of drugs that have high extraction ratios are listed in Box 4-4 (Bigos et al, 2006; Brunton et al, 2010). Other hepatic changes that occur with age that may affect the metabolism of drugs include changes in specific pathways or types of metabolism. The purpose of drug metabolism is generally to make drugs more water soluble for elimination. Phase I metabolism can be described as involving preparatory processes whereby minor molecular modifications are made to drugs. Phase I includes oxidation reduction, demethylation, and hydroxylation. Phase I metabolism is more likely to decrease with age than is phase II metabolism. Drugs that are metabolized by phase I metabolic pathways, including cytochrome P450 (CYP450) enzyme systems, may accumulate in the older adult. Such drugs should be used cautiously and at lower doses in the elderly. Examples of drugs that undergo phase I metabolism include lidocaine, phenytoin, propranolol, and theophylline. If possible, the use of alternative agents within a class of drugs that are metabolized differently (e.g., phase II) should be considered. If these drugs are prescribed in the elderly, lower doses should be used, and patients should be monitored for adverse effects. Examples of drugs whose clearance depends on phase I metabolism are listed in Box 4-5. Drugs that are metabolized by phase II metabolic processes, including conjugation, acetylation, sulfonation, and glucuronidation, show no reported change in clearance with aging (Brunton et al, 2010). Drugs that are metabolized by the liver may undergo reduced metabolism with aging because of changes within the liver itself or changes caused by other disease states. Aging influences associated with declining liver mass, as well as decreased hepatic blood flow, altered nutritional status, other physiologic changes, and diseases such as CHF, may result in the loss of hepatic reserve. Consequently, patients are at increased risk of adverse effects because of competition for the same metabolic enzymes when drugs are added to the existing regimen (Bigos et al, 2006; Brunton et al, 2010). Drug substances that are metabolized and excreted by the liver should be used at a starting dose that is 30% to 40% less than the average dose used in middle-aged adults (Wynne, 2005). Longitudinal studies reflect a great degree of variability in renal function changes with aging. Age-related changes in renal function are the single most important physiologic causative factor in ADRs. Biologic changes that occur in the aging kidney include decreases in the number of nephrons; decreases in renal blood flow, glomerular filtration rate, and tubular secretion rate; and increases in the number of sclerosed glomeruli. In addition, atherosclerotic changes and declining cardiac output decrease renal perfusion by 40% to 50% between the ages of 25 and 65 years. The end result of these changes is noted in creatinine clearance, which is reported to decrease by 10% for each decade after age 40 (Bigos et al, 2006). In addition to the normal reduction in renal function that may occur with aging, chronic diseases including CHF, liver disease, and acute urinary retention from benign prostatic hyperplasia—conditions that lead to dehydration—can also affect renal function and further complicate the required dosing. Creatinine is a muscle byproduct that is almost exclusively removed by the kidney, making it an excellent marker by which to measure renal clearance. Daily creatinine is related to age and serum creatinine concentrations. A drug’s clearance is the amount of blood from which a drug is cleared per unit time. Although creatinine clearance is used to measure renal function, it is important to note that this is only an estimated value. Numerous formulas are available that can be used to calculate the creatinine clearance values, many of which may overestimate or underestimate the patient’s true creatinine clearance. Creatinine clearance is measured by collecting urine for 24 hours. This, unfortunately, may not be practical in many institutions; therefore, we calculate estimated creatinine clearance. In older adults, creatinine clearance calculations may be especially inaccurate because these individuals have very little muscle mass and produce very little creatinine. A patient’s serum creatinine may be reported as low—for example, 0.5 mg/dl (normal, 0.6 to 1.2 mg/dl)—thus reflecting good excretion of creatinine. This information can be highly deceptive and often leads to overestimation of renal function when used with various available formulas. When renally excreted drugs are prescribed for the elderly, calculated creatinine clearance should be used to estimate renal function. Lower drug doses or longer intervals between dosing should be used when renal impairment is evident. Table 4-1 lists drugs that depend on the kidneys for elimination and that therefore may require dose adjustments in the elderly (Brunton et al, 2010). TABLE 4-1 Sample Drugs That May Require Dose Adjustment in the Elderly Because of Renal Impairment Data compiled from Burton ME, Shaw LM, Schentag JJ et al: Applied pharmacokinetics and pharmacodynamics: principles of therapeutic drug monitoring, ed 4, Baltimore, 2005, Lippincott Williams & Wilkins; Duthie EH et al: The practice of geriatrics, ed 4, Philadelphia, 2007, Saunders. A formula that is commonly used to calculate estimated creatinine clearance is the Cockcroft-Gault equation. Unfortunately, the Cockcroft-Gault equation is noted to overestimate the glomerular filtration rate. A simple modification of the Cockcroft-Gault equation has been recommended (DiPiro et al, 2011). This replaces the serum creatinine value with 1 mg/dl if the value is less than 1 mg/dl.
Special Populations
Geriatrics
Functional Assessment
Pharmacokinetic Changes That Affect Drug Therapy
Absorption
Distribution
Biotransformation (Metabolism)
Elimination
Drug Class
Drug Names
Antibiotics
Aminoglycosides, e.g., gentamicin, tobramycin, amikacin
sulfamethoxazole, trimethoprim
β-Lactams: cephalosporins (most), imipenem, ticarcillin
tetracycline
vancomycin
aztreonam
ciprofloxacin, norfloxacin
nitrofurantoin
Antivirals
amantadine, rimantadine
Antineoplastics
methotrexate, bleomycin, nitrosourea, cisplatin
Antifungals
amphotericin B, fluconazole
acyclovir, famciclovir
Analgesics
Opiate analgesics, e.g., meperidine, morphine
Cardiac medications
β-Blockers, e.g., atenolol, nadolol
digoxin
procainamide, bretylium
Angiotensin-converting enzyme inhibitors, e.g., captopril, lisinopril, and others
Other antihypertensives, e.g., clonidine
Diuretics, e.g., thiazides, furosemide, spironolactone
Ulcer medications
Histamine2 blockers: cimetidine, ranitidine, famotidine
Psychoactive medications
lithium, atypical antipsychotics, antipsychotics
Other agents
allopurinol, acetazolamide, chlorpropamide, gold sodium thiomalate, metoclopramide
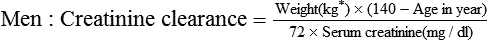
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Special Populations: Geriatrics
Only gold members can continue reading. Log In or Register to continue
