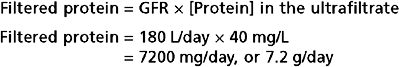CHAPTER 33 Solute and Water Transport along the Nephron: Tubular Function
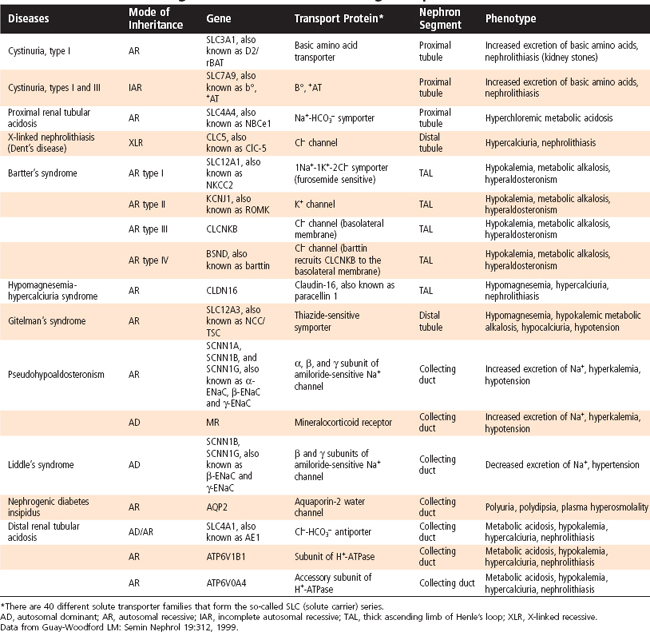
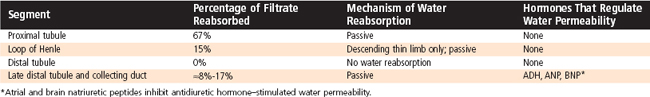
The formation of urine involves three basic processes: (1) ultrafiltration of plasma by the glomerulus, (2) reabsorption of water and solutes from the ultrafiltrate, and (3) secretion of selected solutes into tubular fluid. Although an average of 115 to 180 L/day in women and 130 to 200 L/day in men of essentially protein-free fluid is filtered by the human glomeruli each day,* less than 1% of the filtered water and sodium chloride (NaCl) and variable amounts of other solutes are excreted in urine (Table 33-1). By the processes of reabsorption and secretion, the renal tubules modulate the volume and composition of urine (Table 33-2), which in turn allows the tubules to precisely control the volume, osmolality, composition, and pH of the extracellular and intracellular fluid compartments. Transport proteins in cell membranes of the nephron mediate the reabsorption and secretion of solutes and water in the kidneys. Approximately 5% to 10% of all human genes code for transport proteins, and genetic and acquired defects in transport proteins are the cause of many kidney diseases (Table 33-3). In addition, numerous transport proteins are important drug targets. This chapter discusses NaCl and water reabsorption, transport of organic anions and cations, the transport proteins involved in solute and water transport, and some of the factors and hormones that regulate NaCl transport. Details on acid-base transport and on K+, Ca++, and inorganic phosphate (Pi) transport and their regulation are provided in Chapters 34 through 36Chapter 35Chapter 36.
Table 33-1 Filtration, Excretion, and Reabsorption of Water, Electrolytes, and Solutes by the Kidneys
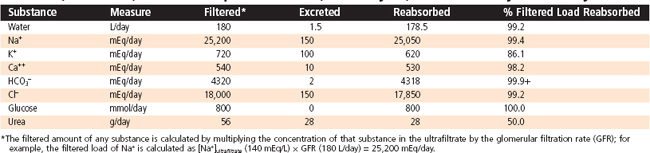
Table 33-2 Composition of Urine
| Substance | Concentration |
|---|---|
| Na+ | 50-130 mEq/L |
| K+ | 20-70 mEq/L |
| Ammonium (NH4+) | 30-50 mEq/L |
| Ca++ | 5-12 mEq/L |
| Mg++ | 2-18 mEq/L |
| Cl− | 50-130 mEq/L |
| Inorganic phosphate (Pi) | 20-40 mEq/L |
| Urea | 200-400 mM |
| Creatinine | 6-20 mM |
| pH | 5.0-7.0 |
| Osmolality | 500-800 mOsm/kg H2O |
| Glucose | 0 |
| Amino acids | 0 |
| Protein | 0 |
| Blood | 0 |
| Ketones | 0 |
| Leukocytes | 0 |
| Bilirubin | 0 |
The composition and volume of urine can vary widely in the healthy state. These values represent average ranges. Water excretion ranges between 0.5 and 1.5 L/day.
Data from Valtin HV: Renal Physiology, 2nd ed. Boston, Little, Brown, 1983.
SOLUTE AND WATER REABSORPTION ALONG THE NEPHRON
The general principles of solute and water transport across epithelial cells were discussed in Chapter 2.
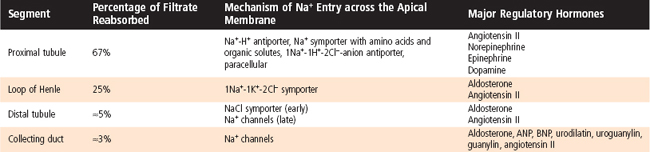
Quantitatively, reabsorption of NaCl and water represents the major function of nephrons. Approximately 25,000 mEq/day of Na+ and 179 L/day of water are reabsorbed by the renal tubules (Table 33-1). In addition, renal transport of many other important solutes is linked either directly or indirectly to reabsorption of Na+. In the following sections, the NaCl and water transport processes of each nephron segment and their regulation by hormones and other factors are presented.
Proximal Tubule
Na+ Reabsorption
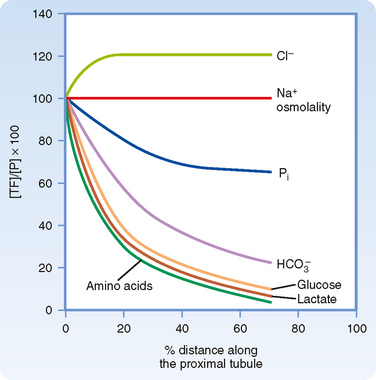
In the first half of the proximal tubule, Na+ uptake into the cell is coupled with either H+ or organic solutes (Fig. 33-1). Specific transport proteins mediate entry of Na+ into the cell across the apical membrane. For example, the Na+-H+ antiporter (Fig. 33-1, A) couples entry of Na+ with extrusion of H+ from the cell. H+ secretion results in reabsorption of sodium bicarbonate (NaHCO3) (see Chapter 36). Na+ also enters proximal cells via several symporter mechanisms, including Na+-glucose, Na+–amino acid, Na+-Pi, and Na+-lactate (Fig. 33-1, B). The glucose and other organic solutes that enter the cell with Na+ leave the cell across the basolateral membrane via passive transport mechanisms. Any Na+ that enters the cell across the apical membrane leaves the cell and enters the blood via Na+,K+-ATPase. In brief, reabsorption of Na+ in the first half of the proximal tubule is coupled to that of HCO3− and a number of organic molecules. Reabsorption of many organic molecules is so avid that they are almost completely removed from the tubular fluid in the first half of the proximal tubule (Fig. 33-2). Reabsorption of NaHCO3 and Na+–organic solutes across the proximal tubule establishes a transtubular osmotic gradient (i.e., the osmolality of the interstitial fluid bathing the basolateral side of the cells is higher than the osmolality of tubule fluid) that provides the driving force for the passive reabsorption of water by osmosis. Because more water than Cl− is reabsorbed in the first half of the proximal tubule, the [Cl−] in tubular fluid rises along the length of the proximal tubule (Fig. 33-2).
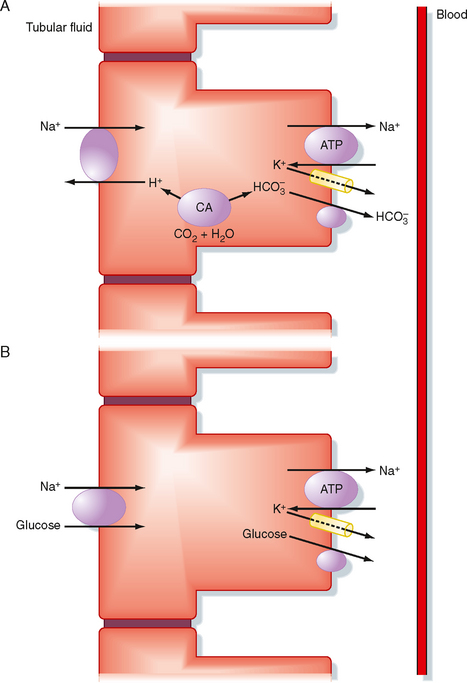
Figure 33-1 Na+ transport processes in the first half of the proximal tubule. These transport mechanisms are present in all cells in the first half of the proximal tubule but are separated into different cells to simplify the discussion. A, Operation of the Na+-H+ antiporter (NHE3) in the apical membrane and the Na+,K+-ATPase and HCO3− transporters, including the Cl−-HCO3− antiporter (AE2) and the 1Na+-3HCO3− symporter (NBC1; see also Chapter 36) in the basolateral membrane mediates reabsorption of NaHCO3. Note that a single HCO3− transporter is illustrated for simplicity. Carbon dioxide and water combine inside the cells to form H+ and HCO3− in a reaction facilitated by the enzyme carbonic anhydrase (CA). B, Operation of the Na+-glucose symporter (SGLT2) in the apical membrane, in conjunction with Na+,K+-ATPase and the glucose transporter (GLUT2) in the basolateral membrane, mediates Na+-glucose reabsorption. Inactivating mutations in the GLUT2 gene lead to decreased glucose reabsorption in the proximal tubule and glucosuria (i.e., glucose in the urine). Though not shown, Na+ reabsorption is also coupled with other solutes, including amino acids, Pi, and lactate. Reabsorption of these solutes is mediated by the Na+–amino acid, Na+-Pi, and Na+-lactate symporters located in the apical membrane and the Na+,K+-ATPase, amino acid, Pi, and lactate transporters located in the basolateral membrane. Three classes of amino acid transporters have been identified in the proximal tubule: two that transport Na+ in conjunction with either acidic or basic amino acids and one that does not require Na+ and transports basic amino acids.
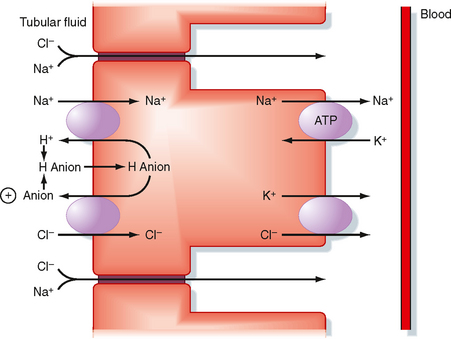
In the second half of the proximal tubule, Na+ is mainly reabsorbed with Cl− across both the transcellular and paracellular pathways (Fig. 33-3). Na+ is primarily reabsorbed with Cl− rather than organic solutes or HCO3− as the accompanying anion because the Na+ transport mechanisms in the second half of the proximal tubule differ from those in the first half. Furthermore, the tubular fluid that enters the second half contains very little glucose and amino acids, but the high [Cl−] (140 mEq/L) in tubule fluid exceeds that in the first half (105 mEq/L). The high [Cl−] is due to the preferential reabsorption of Na+ with HCO3− and organic solutes in the first half of the proximal tubule.
The mechanism of transcellular Na+ reabsorption in the second half of the proximal tubule is shown in Figure 33-3. Na+ enters the cell across the luminal membrane primarily via the parallel operation of an Na+-H+ antiporter and one or more Cl−-anion antiporters. Because the secreted H+ and anion combine in the tubular fluid and reenter the cell, operation of the Na+-H+ and Cl−-anion antiporters is equivalent to uptake of NaCl from tubular fluid into the cell. Na+ leaves the cell via Na+,K+-ATPase, and Cl− leaves the cell and enters the blood via a K+-Cl− symporter in the basolateral membrane.
In summary, reabsorption of Na+ and Cl− in the proximal tubule occurs across paracellular and transcellular pathways. Approximately 67% of the NaCl filtered each day is reabsorbed in the proximal tubule. Of this, two thirds moves across the transcellular pathway, whereas the remaining third moves across the paracellular pathway (Table 33-4).
Water Reabsorption
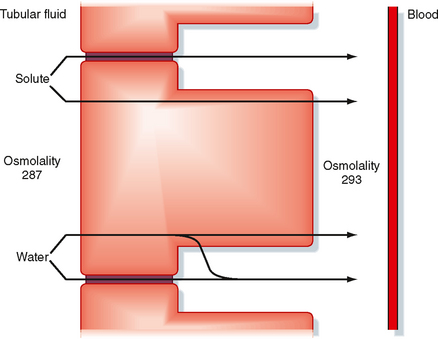
The proximal tubule reabsorbs 67% of the filtered water (Table 33-5). The driving force for water reabsorption is a transtubular osmotic gradient established by reabsorption of solute (e.g., NaCl, Na+-glucose). Reabsorption of Na+ along with organic solutes, HCO3−, and Cl− from tubular fluid into the lateral intercellular spaces reduces the osmolality of the tubular fluid and increases the osmolality of the lateral intercellular space (Fig. 33-4). Because the proximal tubule is highly permeable to water, water is reabsorbed via osmosis. Because the apical and basolateral membranes of proximal tubule cells express aquaporin water channels, water is primarily reabsorbed across the proximal tubular cells. Some water is also reabsorbed across the tight junctions. The accumulation of fluid and solutes within the lateral intercellular space increases hydrostatic pressure in this compartment. The increased hydrostatic pressure forces fluid and solutes into the capillaries.* Thus, water reabsorption follows solute reabsorption in the proximal tubule. The reabsorbed fluid is slightly hyperosmotic relative to plasma. However, this difference in osmolality is so small that it is commonly said that proximal tubule reabsorption is isosmotic (i.e., 67% of the filtered load of solute and water is reabsorbed). Indeed, there is little difference in the osmolality of tubular fluid at the start and end of the proximal tubule. An important consequence of osmotic water flow across the proximal tubule is that some solutes, especially K+ and Ca++, are entrained in the reabsorbed fluid and thereby reabsorbed by the process of solvent drag (Fig. 33-4). Reabsorption of virtually all organic solutes, Cl− and other ions, and water is coupled to Na+ reabsorption. Therefore, changes in Na+ reabsorption influence the reabsorption of water and other solutes by the proximal tubule.
Secretion of Organic Anions and Organic Cations
Cells of the proximal tubule also secrete organic cations and organic anions. Secretion of organic cations and anions by the proximal tubule plays a key role in limiting the body’s exposure to toxic compounds derived from endogenous and exogenous sources (i.e., xenobiotics). Many of the organic anions and cations (Tables 33-6 and 33-7) secreted by the proximal tubule are end products of metabolism that circulate in plasma. The proximal tubule also secretes numerous exogenous organic compounds, including numerous drugs and toxic chemicals. Many of these organic compounds can be bound to plasma proteins and are not readily filtered. Therefore, only a small proportion of these potentially toxic substances are eliminated from the body via excretion after filtration alone. Such substances are also secreted from the peritubular capillary into tubular fluid. These secretory mechanisms are very powerful and remove virtually all organic anions and cations from plasma that enter the kidneys. Hence, these substances are removed from plasma by both filtration and secretion.
Table 33-6 Some Organic Anions Secreted by the Proximal Tubule
| Endogenous Anions | Drugs |
|---|---|
< div class='tao-gold-member'> Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|