Small Cell Carcinoma and Other Neuroendocrine Tumors
Gladell P. Paner, MD
Rafael E. Jimenez, MD
Key Facts
Terminology
NE tumors of prostate include spectrum of primary prostatic tumors exhibiting NE differentiation; in their pure form, they are analogous to NE tumors of other organ sites
Occur either as pure or admixed with acinar adenocarcinoma
Includes SCC, focal NE differentiation in acinar adenocarcinoma, carcinoid tumor, and LCNEC
Clinical Issues
SCC accounts for 1-5% of all prostate cancers, including SCC admixed with adenocarcinoma
Focal NE differentiation in acinar adenocarcinoma is reported in 10-100% of adenocarcinomas
Carcinoid tumor and LCNEC are exceedingly rare
Serum PSA level variable and may be normal
Prognosis of SCC is poor with mean survival of < 1 year after development of SCC component
Microscopic Pathology
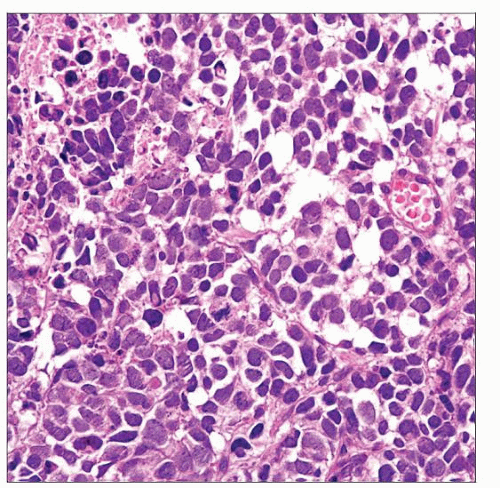
SCC is histologically similar to SCC of lungs
Small blue cell tumor with scant cytoplasm, high nuclear to cytoplasmic ratio, “salt and pepper” chromatin, nuclear molding, single cell necrosis or geographic necrosis, and smearing artifacts
Focal NE differentiation in acinar adenocarcinoma
Characterized by scattered or focal cluster of NE cells blending with adenocarcinoma cellular elements, highlighted by NE markers
Paneth cell-like change may occur
Carcinoid tumor and LCNEC are histologically similar to their pulmonary counterparts
Up to 90% of NE tumors are NE marker (+)
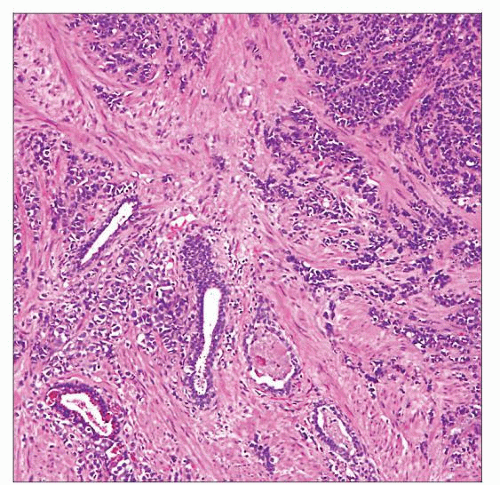 Low-power magnification of SCC involving the prostate shows tumor cells arranged in haphazard nests and trabeculae with infiltration between benign glands. |
TERMINOLOGY
Abbreviations
Small cell carcinoma (SCC)
Synonyms
Small cell neuroendocrine (NE) carcinoma, poorly differentiated NE carcinoma, small cell anaplastic carcinoma, oat cell carcinoma
Definitions
NE tumors of prostate
Spectrum of primary prostatic tumors exhibiting NE differentiation which, in pure form, are analogous to NE tumors of other organ sites
NE tumors occur either as pure or admixed with acinar adenocarcinoma
WHO 2002 classification recognizes 3 forms of prostatic NE tumors
SCC, focal NE differentiation in acinar adenocarcinoma, and carcinoid tumor
SCC
High-grade NE carcinoma consisting of small to intermediate-sized NE tumor cells
Focal NE differentiation in acinar adenocarcinoma
Rare to occasional single or clusters of NE cells present in acinar adenocarcinoma, more often demonstrated by immunohistochemistry
Carcinoid tumor
Well-differentiated NE carcinoma
Large cell NE carcinoma (LCNEC)
High-grade NE carcinoma consisting of large-sized NE tumor cells, often with prominent nucleoli
Recently reported entity
Mixed acinar and SCC
Distinct components, ± juxtaposition, of usual acinar carcinoma and SCC
ETIOLOGY/PATHOGENESIS
Origin
Transdifferentiation or dedifferentiation from non-NE tumor cells
De novo transformation from prostatic NE cells
These are terminally differentiated postmitotic cells that evolved from committed basal cells and share common stem cell origin with acinar and basal cells
These NE cells are believed to play role in growth, differentiation, and homeostatic regulation of secretory processes of prostatic glands
Risk Factors
History of acinar adenocarcinoma, present in about 1/2 of SCC and most carcinoid tumors and LCNEC
Hormonal treatment or androgen deprivation therapy (ADT) for acinar adenocarcinoma
NE cells are devoid of androgen receptors; ADT may lead to clonal propagation
Possible clonal progression and evolution of subset of non-NE tumor cells that have been influenced by ADT
CLINICAL ISSUES
Epidemiology
Incidence
SCC accounts for approximately 1% of all prostate cancers, when SCC admixed with adenocarcinoma are included
Focal NE differentiation in acinar adenocarcinoma is almost ubiquitous, reported in 10-100% of adenocarcinomas, depending on technique employed
Carcinoid tumor and LCNEC are exceedingly rare, described only in isolated case reports and small case series
Poorly differentiated NE carcinomas arise in approximately 10% of prostate cancer patients with androgen-resistant disease following long-term ADT
Age
Tumors occur predominantly in elderly patients
SCC
Mean: 69 years; range: 30-92 years
Presentation
SCC
Rapid onset urinary tract obstruction, such as dysuria, nocturia, or urgency, are main presenting symptoms
Previous diagnosis of adenocarcinoma in 42-67% cases, some with history of prior hormonal therapy
Interval from adenocarcinoma to diagnosis of SCC ranges from 1 to 300 months; mean: 59 months
Lack of clinically evident hormone production in most cases
Paraneoplastic syndromes: Adrenocorticotrophic hormone (ACTH) or antidiuretic hormone (ADH) production, Eaton-Lambert syndrome, and others
Most patients present with extraprostatic extension, large primary tumor masses, advanced stage disease, and distant metastases
Carcinoid tumor
Incidental or may present with hematuria, burning, nocturia, frequency, oliguria, or symptoms of urinary retention
May occur following diagnosis of acinar adenocarcinoma
LCNEC
Most patients with initial diagnosis of acinar adenocarcinoma and prior ADT
Interval from adenocarcinoma to diagnosis of LCNEC ranges from 2 to 12 years; mean: 4.7 years
Advanced stage at time of diagnosis
Clinical and therapeutic significance of distinguishing LCNEC from SCC in prostate is not established
Laboratory Tests
Serum PSA level variable, may be normal
May show significant drop in serum PSA, as NE component predominates over acinar adenocarcinoma component
Serum chromogranin-A and pro-gastrin-releasing peptide levels
May be diagnostically and prognostically useful in prostate cancers with focal NE differentiation
May be useful particularly in androgen-independent cancers
Treatment
Surgery remains mainstream for therapy of SCC
Cisplatin-based chemotherapy has been suggested in SCC, but studies showing significant survival impact are lacking
Prognosis
Prognosis of SCC is poor with mean survival of < 1 year after development of SCC component
No difference in prognosis between pure SCC and SCC admixed with adenocarcinoma
Response to available treatment modalities is poor
Common metastatic sites include bone, liver, lung, and lymph nodes
Prognostic significance of focal NE differentiation in acinar adenocarcinoma is controversial
Some studies show negative effect on prognosis, while some studies show no relationship
Paneth cell-like change in adenocarcinoma does not portend poor prognosis
Prognosis of carcinoid tumor is uncertain because of few cases reported
Some tumors with clinically aggressive behavior may represent prostate adenocarcinoma (carcinoid-like adenocarcinoma)
True carcinoid is suggested to have indolent behavior, although cases reported are limited
Prognosis of LCNEC is similar to SCC
Most common metastatic site is bone; other sites of metastasis include lung, liver, lymph nodes
MACROSCOPIC FEATURES
General Features
Large volume disease frequently, with diffuse involvement of prostatic parenchyma
MICROSCOPIC PATHOLOGY
Histologic Features
SCC
Histologically similar to SCC of lung
Predominantly diffuse sheet-like growth; may form clusters, trabeculae, cords, and single cell patterns
Small blue cell tumor
Scant cytoplasm
High nuclear to cytoplasmic ratio
“Salt and pepper” chromatin
Nuclear molding
Single cell necrosis or geographic necrosis
Smearing artifacts
Brisk mitotic activity
Frequent apoptosis
2 cell types
Oat cell carcinoma consists of small cells (up to 2x size of lymphocytes), pyknotic round to oval nuclei, and indistinct nucleoli
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree