Grade of ACR
Apoptotic bodies per 10 consecutive crypts (n)
Epithelial injury
Lamina propria inflammation
No ACR
≤2
None
Normal components
Indeterminate
3–5
Minimal, usually focal
Minimal, usually localized
Mild
≥6
Mild, usually focal
Mild, usually localized
Moderatea
≥6 and confluentb
Focal crypt dropout, focal mucosal erosion
Moderate, usually extensive
Severea
Variable in residual crypts
Extensive crypt dropout, extensive erosion and/or ulceration
Moderate to severe, extensive
Histological features |
Increased crypt apoptotic activity: three to five apoptotic bodies per ten consecutive crypts |
May show minimal epithelial injury, usually focal, characterized by reactive changes such as loss of mucin, nuclear enlargement and hyperchromasia, cytoplasmic basophilia, mild villous blunting, and others |
Intact surface epithelium |
May show a minimal increase in the number of lamina propria inflammatory cells, consisting predominantly of mononuclear cells. Activated lymphocytes may be present |
Differential diagnosis |
Mild ACR, which may show similar histological findings but with at least six apoptotic bodies per ten consecutive crypts |
Infectious enteritis, which may show increased apoptotic activity but may also show more neutrophils and more severe epithelial injury disproportionate to apoptotic activity. Correlation with laboratory studies, such as serologies, stool studies, cultures, polymerase chain reaction, immunohistochemistry, and others, may be necessary |
Medication effect (see Sect. 6.1.4) |
Potential diagnostic pitfalls |
Diagnosis of indeterminate for ACR implies that features of rejection are present but insufficient for diagnosis of mild rejection and, thus, should not be used in the context of nonspecific inflammation or infection |
Intraepithelial lymphocytes, eosinophils, or neutrophils should not be mistaken for apoptotic bodies |
Histological features |
Increased crypt apoptotic activity: ≥6 apoptotic bodies per 10 consecutive crypts |
May show mild epithelial injury, usually focal, characterized by reactive changes such as loss of mucin, nuclear enlargement and hyperchromasia, cytoplasmic basophilia, mild villous blunting, and others |
Intact surface epithelium |
May show a mild increase in the number of lamina propria inflammatory cells, consisting predominantly of mononuclear cells. Activated lymphocytes, eosinophils, and neutrophils may be present |
Differential diagnosis |
Indeterminate for ACR, which would have similar findings but with <6 apoptotic bodies per 10 consecutive crypts |
Moderate ACR, which may show confluent apoptosis but would also show focal crypt dropout and/or focal erosion of the mucosa |
Infectious enteritis (see Sect. 6.4) |
Medication effect (see Sect. 6.1.4) |
Potential diagnostic pitfalls |
Biopsies from or near the stoma or anastomosis may show nonspecific inflammation, villous architectural change, and epithelial injury, which should not be used to evaluate for rejection |
Intraepithelial lymphocytes, eosinophils, or neutrophils should not be mistaken for apoptotic bodies |
Histological features |
Increased crypt apoptotic activity: more prominent than that seen for mild ACR in general. Apoptosis can be confluent to involve all or the vast majority of the epithelial cells in individual crypts |
Focal crypt withering and/or dropout |
Reactive and regenerative changes |
Moderate increase in the number of lamina propria inflammatory cells, usually extensive, consisting predominantly of mononuclear cells. Activated lymphocytes, eosinophils and neutrophils are usually present. Neutrophilic cryptitis may be seen |
May show focal mucosal erosion |
Differential diagnosis |
Severe ACR, which would show more extensive crypt dropout with more extensive erosion or ulceration |
Infectious enteritis, especially cytomegalovirus (CMV) enteritis with ulceration |
Medication effect (see Sect. 6.1.4) |
Potential diagnostic pitfalls |
Biopsies from or near the stoma or anastomosis may show mucosal erosion, which should not be used to evaluate for rejection |
Focal crypt dropout should not be confused with crushing/squeezing artifact caused by biopsy procedure. Crushing artifact is not accompanied by increased numbers of lamina propria inflammatory cells or increased apoptotic activity in preserved crypts |
Histologic features |
Extensive crypt dropout |
Extensive mucosal erosion and/or ulceration |
Increased crypt apoptotic activity in areas adjacent to erosion/ulceration with residual crypts or in concurrent graft biopsies from other sites |
Reactive and regenerative changes |
Moderate to severe increase in the number of lamina propria inflammatory cells, usually extensive, consisting predominantly of mononuclear cells. Activated lymphocytes, eosinophils and neutrophils are usually present |
Severe exfoliative rejection, a form of severe ACR, shows diffuse ulceration with neutrophilic exudates that involves the entire transplanted bowel |
Differential diagnosis |
Infectious enteritis, especially CMV enteritis with ulceration |
Medication effect (see Sect. 6.1.4) |
Chronic rejection, which can also show broad areas of ulceration, but will also show additional findings (see Sect. 6.2) |
Potential diagnostic pitfalls |
Biopsies from anastomotic site can show features of ulceration, which should not be used to evaluate for rejection |
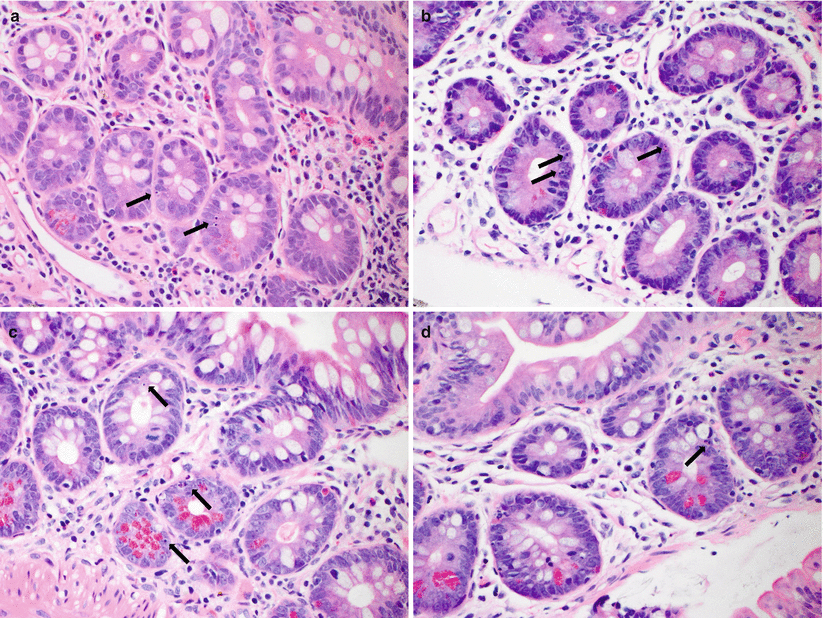
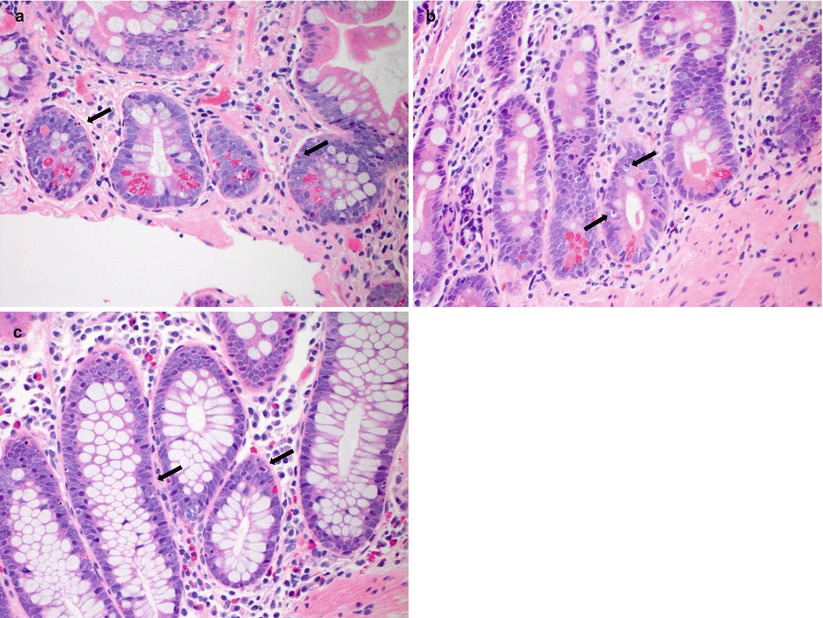
Fig. 6.1
(a–d) Examples of indeterminate for ACR. Minimally increased crypt apoptotic activity, <6 apoptotic bodies per 10 consecutive crypts, which does not meet the criteria for mild ACR. Note the differing morphologies of apoptotic bodies, which can be seen as small, pinpoint basophilic fragments of nuclear debris or as larger exploding nuclear fragments with a rim of clear cytoplasm (arrows, a–d)
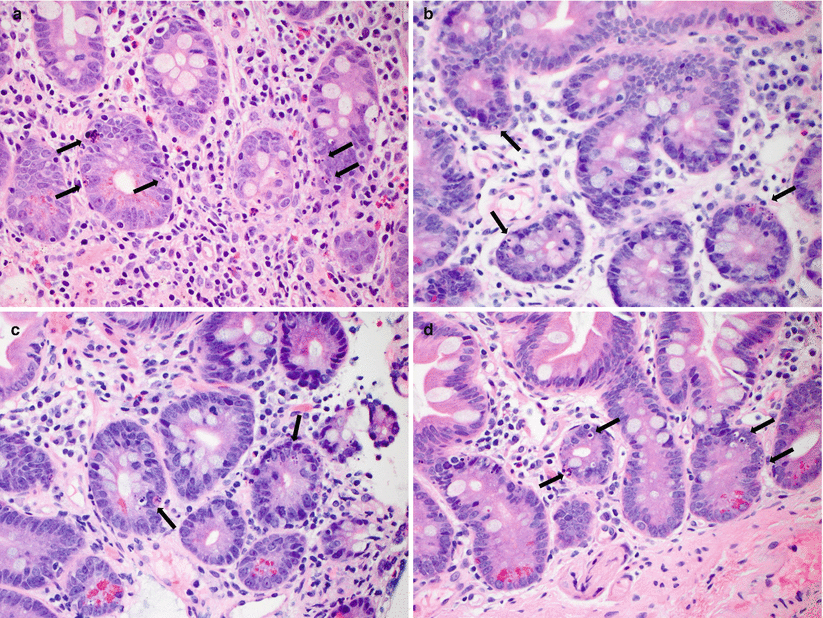
Fig. 6.2
(a–d) Examples of mild ACR. Mildly increased crypt apoptotic activity (arrows, a–d), ≥6 apoptotic bodies per 10 consecutive crypts, which is accompanied by epithelial reactive changes and increased lamina propria inflammatory cell infiltrates. No crypt dropout or erosion/ulceration is noted
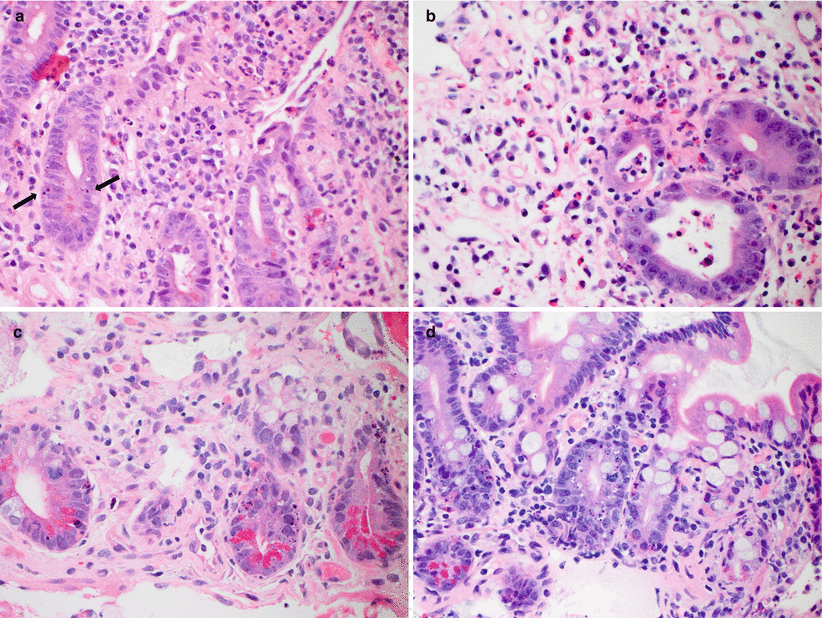
Fig. 6.3
(a–d) Examples of moderate ACR. In addition to increased crypt apoptotic activity (arrows, a), reactive epithelial changes, and increased lamina propria inflammatory cells, moderate ACR is further characterized by more severe mucosal injury including crypt dropout and withering (a), focal surface erosion (b), and confluent crypt apoptosis (c, d)
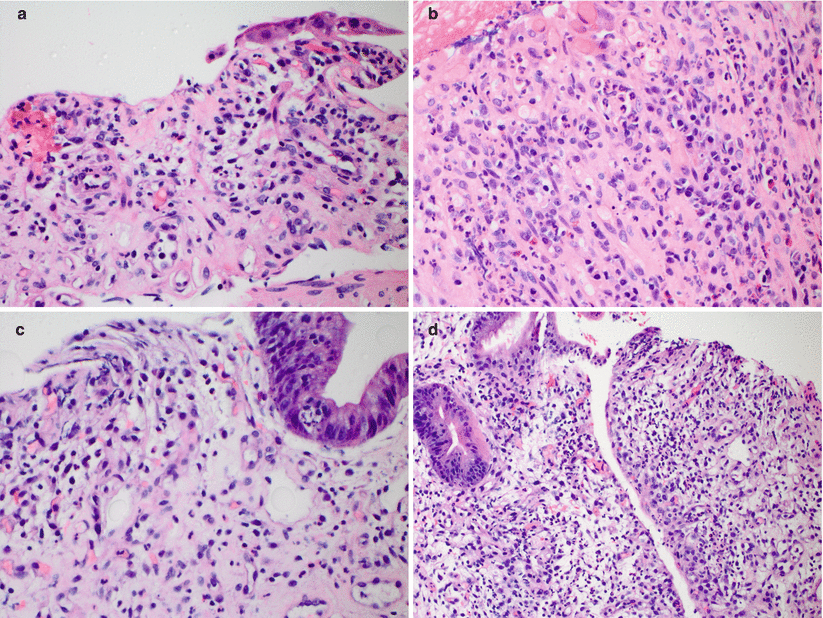
Fig. 6.4
(a–d) Examples of severe ACR. Extensive crypt dropout and ulceration are seen (a, b), with residual epithelial cells showing reactive and regenerative changes as well as increased apoptotic activity (c, d)
6.1.2 General Approach for Evaluation of Small Bowel Graft Biopsies
Routine H&E sections with multiple serial sections are examined.
Low-power examination
Assess adequacy of biopsy: should be deep enough to include the entire mucosa and have at least ten well-oriented crypt bases (in specimens without crypt dropout or ulceration)
Evaluate overall architecture
Intact: no ACR, IND, or mild ACR
Focal crypt dropout or focal erosion: possible moderate ACR
Extensive crypt dropout or ulceration: possible severe ACR
High-power examination
Count crypt apoptotic bodies in crypt bases
May be challenging in tangentially sectioned biopsies, but crypt bases will generally contain Paneth cells
Apoptotic bodies seen in the surface epithelium should not be counted for the purpose of grading ACR
Choose the area with the highest apoptotic density to count
Search for confluent apoptosis, which may involve only a single or a few crypts
Evaluate for viral inclusions and other infectious agents
6.1.3 Subclinical Rejection
Histological findings of ACR seen on surveillance/protocol biopsies in the absence of clinical symptoms, or subclinical rejection (SCR), is a relatively well-established entity in other solid organ transplants and has also been described in SBT [7]. SCR has been reported to account for 17–22 % of biopsies, with the majority showing findings of mild ACR. In their analysis, Takahashi et al. [7] demonstrated that SCR occurring within the first 3 months of transplantation was associated with decreased graft survival.
6.1.4 ACR and Its Differential from Medication Effect (Fig. 6.5)
Mycophenolate mofetil (CellCept) is a commonly used immunosuppressive medication following all types of organ transplantation, including SBT. Its use is limited primarily by gastrointestinal (GI) toxicity, which usually manifests as diarrhea. Recent studies have characterized the histological features of CellCept toxicity, which can affect the entire length of the GI tract and resemble graft-versus-host disease (GVHD), inflammatory bowel disease, acute self-limiting colitis, or ischemic colitis. Erosive or ulcerative enterocolitis can be seen [8–11]. Because increased crypt apoptotic activity is a key diagnostic feature for both ACR and CellCept toxicity (especially for CellCept toxicity with a GVHD-like pattern), differentiating between these two entities can be challenging. Clearly, correlation with the patient’s medication history is essential in these situations. In addition, if concurrent biopsies are taken from native bowel (either small bowel or colon), the distribution of histological findings, in particular apoptotic bodies, can be used to help distinguish between ACR and CellCept toxicity. For example, if increased apoptotic bodies are seen in both graft and native bowel biopsies, this would favor CellCept toxicity because medication effect would be expected to affect both transplanted and native bowels. Conversely, if increased apoptotic bodies are seen only in graft biopsies but are not evident in native bowel biopsies, this would favor ACR because it would be expected to affect only transplanted bowel.